Picea pungens Engelm.
Blue Spruce
Pinaceae -- Pine family
By: Gilbert H. Fechner
From: Burns, Russell M., and Barbara H. Honkala, tech. coords. 1990. Silvics of North America: 1. Conifers; Agriculture Handbook 654. U.S. Department of Agriculture, Forest Service, Washington, DC. vol.2, 877 p.
Blue spruce (Picea pungens) is also called Colorado blue spruce, Colorado spruce, silver spruce, and pino real. It is a slow-growing, long-lived tree of medium size that, because of its symmetry and color, is planted extensively as an ornamental. Because blue spruce is relatively scarce and the wood is brittle and often full of knots, it is not an important timber tree.
Habitat
Native Range
Blue spruce is primarily native to the central and southern Rocky Mountains of the western United States. Its range extends from latitude 33° 50' to 48° 54' N. and from longitude 104° 45' to 114° 00' W.; the Rocky Mountain region in high mountains from southern and western Wyoming, eastern Idaho, south to Utah, northern and eastern Arizona, southern New Mexico, to central Colorado. It has been reported in isolated locations in north-central Montana (83).
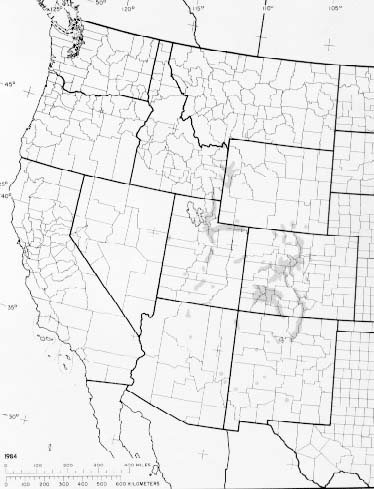
- The native range of blue spruce.
Climate
Blue spruce grows in a climatic zone that is generally cool and humid, with most of the annual precipitation occurring in the summer.
Mean annual temperatures where blue spruce is most commonly found in Colorado and the Southwest range from 3.9° to 6.1° C (39° to 43° F), with a January mean of -3.9° to -2.8° C (25° to 27° F) and a July mean of 13.9° to 15.0° C (57° to 59° F). Mean minimum January temperatures range from -11.1° to 8.9° C (12° to 16° F) and mean maximum July temperatures range from 21.1° to 22.2° C (70° to 72° F). The frost-free period from June to August is about 55 to 60 days (5,69).
Average annual precipitation varies from 460 to 610 mm (18 to 24 in). Winter is usually precipitation-deficient, with less than 20 percent of the annual moisture falling from December through March. Fifty percent of the annual precipitation is rain that falls during the growing season (5,69).
Although blue spruce grows best with abundant moisture, this species can withstand drought better than any other spruce (36). It can also withstand extremely low temperatures (-40° C; -40° F), and it is more resistant to high insolation and frost damage than other associated species.
Soils and Topography
Basic information on soils and landforms needed for silvicultural decisions for blue spruce is limited. Both soils and landforms are very complex. Soils are young and vary widely in texture and physical and chemical properties according to the bedrock from which they originate. Glacial deposits, alluvium from streams, and material weathered in place from country rock are predominant, however (2). The pH is 6.8 to 7.2, neutral to slightly alkaline (21,62). The soils on which blue spruce grows naturally are in the order Mollisols and, to a lesser extent, in the orders Histosols and Inceptisols.
Blue spruce is found on gentle upland and subirrigated slopes, in well-watered tributary drainages, extending down intermittent streams, and on lower northerly slopes. Sites on which blue spruce grows are more moist than those of Rocky Mountain ponderosa pine (Pinus ponderosa var. scopulorum) and warmer than those of Engelmann spruce (Picea engelmannii) and subalpine fir (Abies lasiocarpa) (2,65). In Utah, blue spruce is considered a pioneer tree species on wet soils (21).
Blue spruce is characteristically found at elevations from 1830 to 2740 m (6,000 to 9,000 ft) in its northern range and from 2130 to 3050 m (7,000 to 10,000 ft) in its southern range (27,65).
Associated Forest Cover
Blue spruce is a species of the montane zone in the central and southern Rocky Mountains, where it is the principal species of the Blue Spruce forest cover type (Society of American Foresters Type 216) (27). Blue spruce is also named as a minor associate in four other types: Engelmann Spruce-Subalpine Fir (Type 206), Interior Douglas-Fir (Type 210), Cottonwood-Willow (Type 235), and Interior Ponderosa Pine (Type 237).
Over the bulk of its range, blue spruce is most frequently associated with Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca) and Rocky Mountain ponderosa pine and with white fir (Abies concolor) on mesic sites in the central Rocky Mountains. Blue spruce is seldom found in large numbers, but on streamside sites it is often the only coniferous species present.
Hardwoods associated with blue spruce are most commonly narrowleaf cottonwood (Populus angustifolia), quaking aspen (P. tremuloides), and occasionally balsam poplar (P. balsamifera). Smaller streamside trees and common shrub associates are water birch (Betula occidentalis), mountain alder (Alnus tenuifolia), shrubby cinquefoil (Potentilla fruticosa), common snowberry (Symphoricarpos albus), chokecherry (Prunus virginiana), and species of willow (Salix).
On north-facing slopes, blue spruce, rarely found more than 9 to 12 m (30 to 40 ft) above the drainage bottoms, mixed with Douglas-fir or lodgepole pine (Pinus contorta var. latifolia) (24). At higher elevations, above 2590 m (8,500 ft), blue spruce may mingle with Engelmann spruce, subalpine fir, and quaking aspen on moist sites, or lodgepole pine on drier sites (49).
In its southern range (southwestern Colorado, Arizona, and New Mexico) blue spruce is part of the widespread mixed conifer forest as a component of several diverse habitat types constituting topoedaphic climaxes in stream bottoms and meadow borders. In general, blue spruce dominates habitats that are too warm for Engelmann spruce and subalpine fir and that are wetter than those typically occupied by ponderosa pine. Shrub associates include Rocky Mountain maple (Acer glabrum), western serviceberry (Amelanchier alnifolia), common juniper (Juniperus communis), and Gambel oak (Quercus gambelii), as well as alders and willows on the moister sites (50,65).
In its northern range (northern Wyoming, Idaho, and Montana), blue spruce is found only in scattered locations under established stands of narrowleaf cottonwood and among scattered ponderosa pine, with Engelmann spruce and white spruce (Picea glauca) associated with the species in the extreme north (64,84).
Life History
Reproduction and Early Growth
Flowering and Fruiting- Blue spruce is monoecious. Male strobili develop throughout the living crown of the tree, although they are usually more frequent in the upper one-half of the crown. They commonly develop in whorls of three to five at the base of the current vegetative growth, or singly in subterminal positions (25). Female strobili develop in the upper 10 to 25 percent of the live crown of mature trees. They usually occupy terminal positions on lateral branchlets.
Most male strobili of blue spruce are rose red when they emerge from the buds, but on occasional trees they appear yellowish-green. A single male strobilus, containing 100 sporophylls, may produce about 370,000 pollen grains. The female strobili consist of 175 to 225 scales and thus have a potential to produce 350 to 450 seeds per cone. Pollen is shed in May or June, depending upon altitude.
For a short period of time following emergence from the bud, the scales of the female strobili are a pale greenish color. As peak receptivity is reached, however, the scales of the strobili on most trees become red and are reflexed 90 degrees or more toward the base of the strobilus, which assumes an erect position on the twig. Occasional trees produce yellowish-green strobili. Approximately 2 weeks following initial receptivity, the female strobilus moves from this erect position to about 45 degrees above horizontal. In another week, 50 percent of the cones on a tree are 45 degrees below horizontal to pendent. During the fourth week, all cones become pendent and reach their full size (24,26,28).
Seed Production and Dissemination- Blue spruce is generally considered to be from good to prolific in seed production, yielding full crops of cones every 2 or 3 years (77,84). Some intermediate years are complete failures (24). Seed production begins at approximately 20 years, and optimum seed-bearing age is reached between 50 and 150 years (88). Cones mature in August of the first year; seed shed begins from early to late September, depending on altitude, and continues into the winter (26,77). The seed is wind disseminated, seedfall diminishing rapidly as distance from the source increases; most seeds fall within 90 m (300 ft) of the upwind timber edge (2).
It is unlikely that heavy cone crops will occur in successive years on a single blue spruce tree, because the female strobili occupy terminal positions on lateral branchlets. Such terminal positions are at a minimum in the year following one of high seed production, because once a strobilus is differentiated from an apical meristem, only the strobilus develops at that position during the following growing season. If a whorl of new axillary buds is produced on the branchlet at the base of the developing cone, these buds ordinarily produce vegetative shoots for one season before female strobili are again differentiated. Thus, although blue spruce cones occasionally occupy sessile, axillary positions, the likelihood of heavy seed crops occurring more frequently than every 2 years is very remote (24).
Seedling Development- Seeds of blue spruce germinate on a variety of media, although natural reproduction is mostly confined to exposed mineral soil with side shade and overhead light in the vicinity of seeding trees. Natural reproduction is scanty, probably because the lightweight seed is prevented from coming into contact with mineral soil by the dense herbage, grass, or other ground-cover vegetation that is usually abundant in the habitat of the species (84).
Seeds of blue spruce were once thought to show embryo dormancy. It is now known, however, that blue spruce seeds germinate promptly and completely without prior stratification, under a wide range of temperatures, with or without light (46). Germination is epigeal (77).
In most parts of the blue spruce range natural germination of seed takes place in the spring or summer following dispersal and is dependent on adequate precipitation (51).
Spring and early summer drought periods occur regularly in the Southwest. Although soils of the mixed conifer forest are wet at the end of winter from melting snows, these drought periods during the growing season create soil moisture deficits that are critical to initial seedling survival. Fall moisture deficits common over the remainder of the range are less limiting to seedling establishment and usually do not kill seedlings established for 2 years or more except on severely affected sites (2,52).
Blue spruce seedlings are more sensitive to day temperatures between 13° and 31° C (55° and 88° F) than to night temperatures between 7° and 25° C (45° and 77° F) (86).
Under greenhouse conditions, blue spruce seedlings are affected by supplemental light. They grow continuously when exposed to photoperiods exceeding 16 hours and enter dormancy within 4 weeks under photoperiods of 12 hours or less. Dormancy is prevented under 12-hour photoperiods by 2-hour light breaks of red light (1.70 µw/cm² @ 650 nm) or high intensity white light (2,164.29 µw/cm² @ 400 to 800 nm) given in the middle of the 12-hour night (94), or by one-minute light breaks every 30 minutes throughout the night (85).
The establishment of blue spruce seedlings under natural conditions is probably benefited by moisture availability and shading, which prolong snow and soil moisture in late spring.
Early growth of blue spruce seedlings is very slow. In a Michigan nursery study, the tallest of 50 populations averaged 15.7 cm (6.2 in) at 2 years (40). In North Dakota, the tallest of seven sources was 58.4 cm (23.0 in), 5 years after outplanting (18). Similarly, in a plantation in the southern range, trees were 48.5 to 59.2 cm (19.1 to 23.3 in) tall after five growing seasons (53).
Vegetative Reproduction- Natural vegetative reproduction of blue spruce has not been reported. The species does not sprout from the stump or root, but the development of epicormic branches on the trunk is common. Grafting and air-layering have been practiced successfully for many years to perpetuate desired horticultural varieties (32,60,63, 74,91). Success has also been achieved through the rooting of hardwood or greenwood stem cuttings, especially in sand-peat-soil media, or hydroponically (56,79,81,93).
Sapling and Pole Stages to Maturity
Growth and Yield- Blue spruce is apparently a long-lived tree, surviving up to 600 years or more. Diameter growth is slow; trees 10 to 13 cm (4 to 5 in) in d.b.h. may be 125 to 135 years old; at 46 to 56 cm (18 to 22 in), they may be 275 to 350 years of age (84). The "1982 National Register of Big Trees" lists the largest blue spruce as 154.4 cm (60.8 in) in d.b.h. and 38.4 m (126 ft) tall, on the Gunnison National Forest, CO.
Few growth and yield data are available for blue spruce. In one study, in a mixed conifer forest in east-central Arizona, blue spruce was found to constitute a total of 0.7 m²/ha basal area (3.05 ft²/acre) of a total of 40.8 m² (177.7 ft²). The 728-ha (1,800-acre) forest consisted of Douglas-fir (31.4 percent), quaking aspen (15.9 percent), white fir (14.5 percent), ponderosa pine (14.1 percent), Engelmann spruce (13.5 percent), southwestern white pine (Pinus strobiformis) (5.6 percent), corkbark fir (Abies lasiocarpa var. arizonica) (3.3 percent), and blue spruce (1.7 percent). In this study, the annual basal area growth for blue spruce was found to be 2.9 percent, greater than that of any other species except corkbark fir, which was 3.7 percent per year (22). The total basal area growth for blue spruce, 0.008 m² (0.088 ft²) per year, was distributed as shown in table 1.
|
D.b.h. class |
Percent
of stand |
Incremental growth |
|
| m²/ha | ft²/acre | ||
| 0.3 to 17.5 cm
0.1 to 6.9 in |
4 |
0.004 | 0.0 |
| 17.8 to 27.7 cm
7.0 to 10.9 in |
18 |
0.001 | 0.006 |
| 27.9 to 42.9 cm
11.0 to 16.9 in |
18 |
0.001
|
0.006 |
| 43.2 to 58.2 cm
17.0 to 22.9 in |
10 |
0.001 | 0.004 |
| 58.4 cm and larger 23.0 in and larger | 5 |
- | 0.002 |
| Total | 100 | 0.007 | 0.035 |
Rooting Habit- Young seedlings of blue spruce are shallow rooted, with roots penetrating the soil only about 6.4 cm. (2.5 in) during the first year (50). Although blue spruce tissue is not damaged much by freezing, seedling losses can result from frost heaving. Shade in late spring and early fall minimizes such frost-heaving losses (2,69).
Even in mature trees, the root system of blue spruce is relatively shallow, compared to that of Douglas-fir and ponderosa pine, adapting it to the moist site on which it usually grows. In spite of the shallow root system, blue spruce is decidedly windfirm. (36).
Pruning roots of blue spruce 5 years before transplanting doubles the total root surface area of 2-meter-tall trees at transplanting time. It also increases the concentration of the root system within the dripline from 40 to 60 percent, an advantage in landscape plantings (90).
Reaction to Competition- Blue spruce is classed as intermediate in tolerance of shade, the middle of five tolerance categories for western conifers. It is less tolerant than subalpine fir, Engelmann spruce, and white fir; it is similar in tolerance to, or slightly more tolerant than, Douglas-fir; it is more tolerant than southwestern white pine, ponderosa pine, lodgepole pine, Rocky Mountain juniper, quaking aspen, or its other moist-site hardwood associates (4,27,52,62).
On cool sites, a dense or moderately dense canopy favors regeneration of subalpine fir, blue spruce, white fir, and Engelmann spruce, to the exclusion of Douglas-fir. On warm sites, an open canopy favors ponderosa pine, whereas a moderate canopy favors Douglas-fir (92).
Blue spruce occurs in various seral stages, from pioneer to climax, in 32 currently recognized habitat types (28). The exact successional status depends on the location within its geographic range and on its immediate associates. For example, in the Southwest, blue spruce represents a topo-edaphic climax, one in which environmental factors compensate for one another (17); here it reproduces and is present in all sizes, along stream banks, in well-watered tributaries, on gentle lower slopes, and in forest borders of grassy meadows. On these sites, ponderosa pine and Douglas-fir may be long-lived seral species, white fir and southwestern white pine may occur as minor seral species, and subalpine fir may be of accidental occurrence (58,65). Blue spruce may also form climax stands with Engelmann spruce on slopes and in drainages at higher elevations and with Douglas-fir and white fir (1) on lower slopes and north aspects at lower elevations (65). Blue spruce may be a minor seral species in white fir- and subalpine fir-dominated forests on cooler sites (58), and it may constitute a pioneer species on wet sites (21).
In Utah, blue spruce is a climax species in three distinct environments: gentle to steep mountain slopes, floodplains and valley bottoms at lower elevations, and montane sites on alluvium or aqueaceous north-aspect deposits (23). Almost exclusively, sites that support climax stands of blue spruce have parent materials of limestone or calcareous sandstone. Thus, blue spruce probably constitutes an edaphic climax on these sites (62,71). On Utah sites, quaking aspen is the prinicipal seral species, except in the Uinta Mountains, where the seral role is assumed by lodgepole pine. At the higher altitudes in Utah, blue spruce becomes a minor seral species to subalpine fir (71).
Damaging Agents- Several insects are known to attack developing cones and seeds of blue spruce, but damage caused by insects is not heavy (55). The spruce seed chalcid (Megastigmus piceae) is found throughout the range of the host. Larvae of the spruce seed moth (Laspeyresia youngana) and the cone cochylid (Henricus fuscodorsana) bore through cone scales near the axis of the cones, destroying both scales and up to 10 percent of the seeds. Larvae of the spruce coneworm (Dioryctria reniculelloides) mine young cones in addition to feeding on tender terminal growth and its foliage (34,45,54).
In addition to those attacking developing cones and seeds, other insects occasionally damage blue spruce (34). The larvae of the western spruce budworm (Choristoneura occidentalis) feed on old needles in late April, then mine developing buds and defoliate new tree growth (59). Heavy, repeated attacks kill the tree.
Less serious damage can be caused by the spruce needle miner (Taniva abolineana), and another needle miner, Coleotechnites piceaella (34,43,54). The Cooley spruce gall aphid (Adelges cooleyi) and the pine leaf aphids (Pineus pinifoliae and Pineus similis) cause the formation of cone-shaped galls. The former may be of consequence on seedlings and saplings.
Other insects that attack blue spruce are the green spruce aphid, Cinara fornacula, and the related Cinara coloradensis, which feed on terminal twigs, as does the white pine weevil (Pissodes strobi). Twig beetles, Pityophthorus spp., may attack injured trees. Dendroctonus rufipennis, the spruce beetle, is also found on blue spruce. Ips pilifrons, an engraver beetle which attacks recently downed trees, may deprive the spruce beetle of favorable breeding places, thereby reducing the threat of a spruce beetle outbreak (34,72). Secondary insects are Dryocoetes affaber and the four-eyed spruce beetle (Polygraphus rufipennis). Ambrosia beetles, Gnathotrichus sulcatus, and Trypodendron bivattatum, and the golden buprestid (Buprestis aurulenta), a flatheaded borer, attack the wood.
The rust Chrysomyxa pirolata infects the cones of blue spruce. Seed production is not greatly affected by this disease, however, although malformation of the cones may interfere with seed dispersal (67). Seed viability in rust-infected cones may be reduced, but seeds are not totally destroyed.
A variety of diseases also attack seedlings, leaves, stems, and roots of blue spruce. Damping-off, caused by Phytophthora cinnamomi, kills new seedlings, as does the cylindrocladium root rot, caused by Cylindrocladium scoparium (11,48). Nematodes may reduce root growth of seedlings in nurseries (30,37). Low seedling vigor is also caused by the root lesion nematode, Pratylenchus penetrans (48), and snow molds may cause nursery losses during seasons of heavy snow (82).
Leucocytospora kunzei (Syn.: Cytospora kunzei) is widespread in northeastern United States and may cause cankers on one-fourth to one-half of the branches of blue spruce. Although usually not fatal, branch loss dramatically reduces the aesthetic value of landscape trees (35,73). Phomopsis occulta causes a tip blight on blue spruce; it is characterized by downward curling and necrosis of expanding shoots, where stem cankers and sap exudate commonly occur (78). Western spruce dwarf mistletoe (Arceuthobium microcarpum) causes mortality in infected stands two to five times greater than in healthy stands, and heavily infected trees may show a 10-year volume loss of up to 40 percent (61).
Three species of Chrysomyxa cause needle rusts and moderate amounts of shedding of new needles on blue spruce. Another needle cast fungus, Rhizosphaera kalkhoffii, damages Christmas tree plantations of blue spruce in the Midwest and the East. Serious damage is not associated with natural stands of this species although the disease was first reported on blue spruce in its native range in Arizona (44,68,89). Chrysomyxa arctostaphyli causes the perennial yellow witches' broom on blue spruce branches; Arctostaphylos uva-ursi, the common kinnikinnik, serves as host of stage 3 of the fungus (70). Armillaria mellea and Inonotus tomentosus both cause root rot, and Phellinus pini, Fomitopsis pinicola, Climacocystis borealis' and Polyporus caesius are common heart rots (48).
Special Uses
Blue spruce is valued mainly for its appearance. Shortly after the species was discovered in 1861, writers described it as "a finely shaped tree" and "the most beautiful species of conifer," alluding to the symmetrical, pyramidal form and the glaucous, bluish or silvery-gray foliage that some trees of the species display. The needle coloration, caused by the presence of surface waxes (76), apparently intensifies with tree age (13,14). These traits of symmetry and blue or silver-gray cast, so common in horticultural plantings, are only occasionally found in natural stands. In nature, trees with similar color tend to occur in small, local populations, suggesting genetic control of the color trait.
Blue spruce is widely used as an ornamental, not only in the United States, but in Europe, where it was introduced late in the 19th century. At least 38 cultivars of blue spruce have been named, based primarily on leaf coloration and crown form (3,19) (table 2). Although young blue spruce usually show a pronounced layering of stiff branches, which give it a distinct pyramidal form, the branches begin to droop and the crown becomes thin and irregular as the tree ages. The trunk tapers rapidly, and epicormic shoots commonly develop, giving the tree a ragged appearance. Blue spruce is prized as a Christmas tree, and plantations have been established in its native range and in north-central and northeastern United States.
| Cultivar | Characteristics |
| 'Argentea' Rosenthal | Silvery white |
| 'Aurea' Niemitz | Golden yellow |
| 'Bakeri' Bailey | Deep bluish white, long-leaved |
| 'Caerulea' Beissner | Bluish white |
| 'Compacta' Rehder | Dwarf, compact, densely flat-topped |
| 'Glauca' Beissner
|
Bluish green; collective name for all glaucous-leaved cultivars |
| 'Glauca Pendula' Koster ex Beissner | Pendulous, bluish leaves, strongly sickle-shaped |
| 'Hoopsii' Hoops ex F.J. Grootend | Dense, pyramidal; leaves very silvery |
| 'Hunnewelliana' Hornibr. | Dwarf, dense, pyramidal; leaves pale green |
| 'Koster' Boom
|
Pyramidal, pendulous-branched, with main branches almost horizontal; leaves bluish white to silvery white |
| 'Moerheimi' Ruys
|
Pyramidal, slender, dense, compact; leaves deep blue |
| 'Thomsen' Thomsen
|
Pyramidal; leaves whitish to silvery blue, long |
| 'Viridis' Regel | Dull green |
Genetics
Population Differences
In a study of seven blue spruce provenances from Arizona, Colorado, Utah, and Wyoming, grown in North Dakota, 5-year survival varied from 22 percent for the Targee National Forest, WY, source to 96 percent for an Ashley National Forest, UT, source (18). In the same study, height differed significantly among the sources; the two sources from Ashley National Forest represented the tallest (57.3 cm; 22.6 in) and the shortest (37.5 cm; 14.8 in). No latitudinal or altitudinal pattern of survival, growth, or frost resistance seemed apparent.
In a Michigan nursery study of progenies from 50 populations collected throughout its range, 2-year-old blue spruce seedlings from Colorado, New Mexico, and Arizona grew more rapidly than those from Utah, Wyoming, or Montana. The average heights of the 10 tallest populations ranged from 18.8 to 16.1 cm (7.4 to 6.3 in) (40).
Variation in foliage color is apparently under strong genetic control (15), although the mechanism of inheritance is not presently known. Because there is no consistency in blue color from any one source, color variation is a characteristic to expect with seed-produced trees (47). Two-year-old progenies from Arizona and New Mexico seed sources show a much higher incidence of "blueness" than those from other areas (40). However, little or no difference has been detected between seedlings with glaucous (bluish) or non-glaucous (greenish) needles in photosynthetic rate, transpiration rate, and moisture retention (75). These studies suggest that genetic variation in natural populations of blue spruce does not conform to a clinal pattern. Rather, the pattern appears to be ecotypic, with considerable stand-to-stand variation and individual tree variation.
Significant variation exists among populations in the concentration of terpenes derived from cortical tissue. Five populations, each consisting of 10 selected seed trees, differed significantly in the concentration of each of eight monoterpenes in a Michigan study. Although the total percentages of the eight monoterpenes were similar among the populations, the Utah, Colorado, and Wyoming populations were distinct from the New Mexico and Arizona populations due to percentages of specific monoterpenes. For example, the average percentage of a-pinene was 14.3 for the three northern populations and 8.5 for the two southern ones, whereas b-phellandrene averaged 0.58 percent for the northern populations and 0.89 percent for the southern populations (39).
Large differences in monoterpene yield exist in xylem, bark, and needles of individual blue spruce trees, and variation in terpene yield among trees is significant. The concentration of the terpenes in the needles and xylem varies with crown position, the yield increasing with tree height in the xylem and decreasing with tree height in the needles. These yields are correlated with the proportions of resin canals in the respective tissues (66).
Several investigators have reported different results in blue spruce seedlings grown under accelerated greenhouse conditions (20,39,40,41). In a recent study, height growth of 75 single-tree Colorado sources, grown under accelerated greenhouse conditions, varied significantly among six seed zones but not among families within a seed zone. Seed zone averages ranged from 22.2 cm for the tallest to 14.2 cm for the shortest during the 140-day test period (20).
In their reports of a rangewide provenance study of blue spruce conducted in Michigan, investigators noted that the southern sources of blue spruce did not grow as well under accelerated greenhouse conditions as did the northern sources (6,8,41). In contrast, in the Colorado study, southern Colorado sources generally outgrew the northern Colorado sources.
It is interesting to note that in field plantations subsequently established in several midwestern states and Quebec with their blue spruce sources, the Michigan investigators observed a reversal of the variation patterns that they had observed in the greenhouse. In the field plantations, the southern sources outgrew the northern sources (9,10,87). Thus, growth of the seedlings studied in Colorado in the greenhouse followed much the same patterns as the seedlings that were grown outdoors in the Michigan studies.
In only a single study has the date of bud set been recorded in blue spruce. Within latitudinal groups in Colorado, bud set varied with elevation of the seedling seed source, the high-elevation sources setting bud much sooner than the low-elevation sources (20). Some investigators (6,87) have found no consistent pattern or date of bud break in the 400 widely distributed sources of blue spruce studied. And others (10) found that bud break was variously related to longitude but not to elevation. Yet the results of the Colorado study, based on relatively intensive elevational sampling, show a relationship between latitude and elevation of seed origin and the date of bud set.
Thus, whereas research results support the notion that natural variation of most parameters that have been studied in blue spruce conforms to a discontinuous pattern geographically (18,20,39,40,87), variation in date of bud set conforms to a local altitudinal clinal pattern (20).
Hybrids
From studies of morphological features of blue spruce and Engelmann spruce, it has been concluded that these two species do not hybridize in nature, although no morphological character absolutely separates the two (16). Considerable overlap in cone size has been found; Engelmann spruce cones vary from 2.8 to 5.8 cm (1.1 to 2.3 in) and blue spruce cones vary from 4.5 to 10.7 cm (1.8 to 4.2 in) in neighboring populations measured in northern Colorado (33). Cone and seed characteristics are often found to be indistinguishable (40).
Controlled crosses between blue spruce and Engelmann spruce obtained up to 2 percent sound seed set when Engelmann spruce was the female parent (29). The reciprocal cross was also successful. Only occasional embryos developed following crosses between the two species, but, more frequently, reproductive failure occurred prior to embryo formation (57).
Much overlap between blue spruce and Engelmann spruce in cortical monoterpene content has also been observed, although species differences in the quantity of several of the compounds are statistically significant. Oleoresins of blue spruce contain higher levels of tricyclene, (a-pinene, camphene, and bornyl acetate, whereas Engelmann spruce oleoresins contain higher levels of b-pinene, 3-carene, terpinolene, and several unknown compounds (80).
These and other results (42) indicate that hybridization between blue spruce and Engelmann spruce is possible. This might account for the various intergrades between blue spruce, white spruce, and Engelmann spruce that have been reported in Montana (83).
Information on inheritance patterns for certain characteristics of blue spruce, although somewhat inconclusive, is provided by results of half-sib and full-sib progeny studies involving that species. For example, in a Canadian study (13,14), inheritance of needle coloration was investigated using such controlled crosses. A qualitative rating scale of one (green) to four (silvery blue) was used for comparison. Although the proportion of blue seedlings was not significantly related to the blue color ratings of their open-pollinated parents, the needle-color ratings of 10-year progeny were related to those of their self-pollinated parents (r = 0.83). One selfed tree produced 94 percent blue progeny.
As is true for certain other coniferous species, albinism in blue spruce is apparently controlled by a single gene. The proportion of normal (green) to albino seedlings derived from self-pollinated seeds of two different trees produce a good fit to a 3:1 ratio, suggesting heterozygosity for a simple lethal factor (12).
In Michigan studies, hybrid progeny from crosses between white spruce and blue spruce showed a slight, but nonsignificant, increase in germination rate over the parental half-sib progeny, and at 42 weeks, needle length was intermediate between those of the parental progeny. Although the hybrid progeny as a group displayed intermediacy in 3-carene biosynthesis ability between the two parents, individual-tree values showed genetic segregation in the open-pollinated (half-sib) blue spruce progeny and uniformity in the open-pollinated (half-sib) white spruce progeny (42). Yet, the range of values for 3-carene biosynthesis ability is controlled by a single pair of alleles, as had been shown for western white pine (Pinus monticola) (38). However, when natural populations of blue spruce were studied for this characteristic, allele frequencies for the 3-carene gene did not conform to expected values in Colorado and New Mexico populations, although they did conform to expected single-gene frequencies in the Utah, Arizona, and Wyoming populations (39). These apparent discrepancies could be artifacts of sample size or other unknown factors.
Whereas the initiation date of germination of hybrid seed has been found to be intermediate between parental (half-sib) seed of blue spruce and Engelmann spruce, cotyledon number, mean day of total germination, and hypocotyl color tend to be similar to those of female parent (29). That cotyledon number is under strong maternal control, as it also is in white spruce (31), is supported by a recent study, in which cotyledon number differed significantly (P = .001) between half-sib Colorado families but not within those families (20).
From studies of controlled crosses among white spruce, blue spruce, and red spruce (Picea rubens), F2 progeny of white spruce x blue spruce crosses were found to be much stunted in height and in needle length (7). Further results of findings among these species are summarized in table 3.
| Spruce combination | Character response |
| (White x blue) x white (backcross) | - Similar to white spruce in all measured characters. |
| (White x blue) x blue (backcross)
|
- Similar to blue in 6-month, height, needle curvature,
and 3-carene
concentration.
- Similar to white in needle serrations. - Intermediate in b-pinene concentration |
| (White x blue) x red (trihybrid)
|
- Similar to red in needle serrations, limonene
concentration, and needle curvature.
- Similar to white x red in needle color. - Similar to white x blue in 3-carene and b-pinene concentrations. |
In summary, it would appear that for most needle, chemical synthesis, and germination characteristics that have been studied in blue spruce, the gene action is quantitative. Exceptions to this seem manifest in the biosynthetic ability of 3-carene and in the production of albino seedlings, which may be single-gene controlled, and cotyledon number, hypocotyl color, and mean germination date, which may be under strong maternal influence in that species.
Literature Cited
- Alexander, Billy G., Jr., Frank Ronco, Jr., E. Lee Fitzhugh, and John A. Ludwig. 1984. A preliminary classification of forest vegetation types on the Lincoln National Forest, New Mexico. USDA Forest Service, General Technical Report RM-104. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 29 p.
- Alexander, Robert R. 1974. Silviculture of central and southern Rocky Mountain forests. USDA Forest Service, Research Paper RM-120. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 36 p.
- Bailey, L. H., and E. Z. Bailey. 1976. Hortus third. Macmillan, New York. 1290 p.
- Baker, Frederick S. 1949. A revised tolerance table. Journal of Forestry 47(3):179-181.
- Bates, Carlos C. 1924. Forest types in the central Rocky Mountains as affected by climate and soils. U.S. Department of Agriculture, Bulletin 1233. Washington, DC. 152 p.
- Bongarten, B. C. 1978. Genetic and environmental variation in shoot growth and other traits of blue spruce (Picea pungens). Thesis (Ph.D.), Department of Forestry, Michigan State University, East Lansing. 107 p.
- Bongarten, Bruce C., and James W. Hanover. 1982. Hybridization among white, red, blue, and white x blue spruces. Forest Science 28(l):129-134.
- Bongarten, B. C., and J. W. Hanover. 1985. Accelerating seedling growth through photoperiod extension for genetic testing: A case study with blue spruce. Forest Science 31(3):631-643.
- Bongarten, B. C., and J. W. Hanover. 1986a. Genetic parameters of blue spruce (Picea pungens) at two locations in Michigan. Silvae Genetica 35(2-3):106-112.
- Bongarten, B. C., and J. W. Hanover. 1986b. Provenance variation in blue spruce (Picea pungens) at eight locations in northern United States and Canada. Silvae Genetica 35(2-3):67-74.
- Cordell, Charles E., and Darroll D. Skilling. 1975. Cylindrocladium root rot, Cylindrocladiurn scoparium Morgan. In Forest nursery diseases in the United States. p. 23-26. Glenn W. Peterson and Richard S. Smith, Jr., eds. U.S. Department of Agriculture, Agriculture Handbook 470. Washington, DC.
- Cram, W. H. 1983a. Albinism and natural selfing in Picea pungens. Canadian Journal of Plant Science 63:1097- 1098.
- Cram, W. H. 1983b. Performance of Picea pungens in southern Saskatchewan. Forestry Chronicle 59:146-147.
- Cram, W. H. 1984a. Some effects of self-, cross-, and open-pollinations in Picea pungens. Canadian Journal of Botany 62:392-395.
- Cram, W. H. 1984b. Needle color and vigor of inbred progenies of Picea pungens. HortScience 19(l):125-126.
- Daubenmire, R. 1972. On the relation between Picea pungens and Picea engelmannii in the Rocky Mountains. Canadian Journal of Botany 50 (4):733-742.
- Daubenmire, R., and Jean B. Daubenmire. 1968. Forest vegetation of eastern Washington and northern Idaho. Washington Agricultural Experiment Station Technical Bulletin 60. Pullman, WA. 104 p.
- Dawson, David H., and Paul 0. Rudolf. 1966. Performance of seven seed sources of blue spruce in central North Dakota. USDA Forest Service, Research Note NC-5. North Central Forest Experiment Station, St. Paul, MN. 4 p.
- den Ouden, P., and B. K. Boom. 1965. Manual of cultivated conifers. Martinus Nishoff. The Hague, Netherlands. 526 p.
- Diebel, Kenneth E., and Gilbert H. Fechner. 1988. Natural variation among seedlings from Colorado sources of blue spruce. Western Journal of Applied Forestry 3(4):106-109.
- Dixon, Helen. 1935. Ecological studies on the high plateaus of Utah. Botanical Gazette 97(2):272-320.
- Embry, Robert S., and Gerald J. Gottfried. 1971. Basal area growth of Arizona mixed conifer species. USDA Forest Service, Research Note RM-198. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 3 p.
- Erdman, Kimball S. 1970. Distribution of the native trees of Utah. Brigham Young University Science Bulletin, Biological Series 9(3). Provo, UT. 34 p.
- Fechner, Gilbert H. 1964. The reproductive cycle of Picea pungens Engelmann. Thesis (Ph.D.), University of Minnesota, St. Paul. 199 p.
- Fechner, Gilbert H. 1973. The microsporangium and microsporogenesis in Picea pungens. Department of Forest and Wood Sciences, Colorado State University, Fort Collins, CO. 12 p.
- Fechner, Gilbert H. 1974. Maturation of blue spruce cones. Forest Science 20(l):47-50.
- Fechner, Gilbert H. 1980. Blue spruce-216. In Forest cover types of the United States and Canada. p. 95-96. F. H, Eyre, ed. Society of American Foresters, Washington, DC.
- Fechner, Gilbert H. 1985. Silvical characteristics of blue spruce. USDA Forest Service, General Technical Report RM-117. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 19 p.
- Fechner, Gilbert H., and Roger W. Clark. 1969. Preliminary observations on hybridization of Rocky Mountain spruces. In Proceedings of the Committee on Forest Tree Breeding in Canada. 11:237-247. Department of Fisheries and Forestry of Canada, Ottawa, Ontario. 290 p.
- Ferris, J. M., and A. T. Leiser. 1965. Control of nematodes associated with blue spruce. Plant Disease Reporter 49(l):69-71.
- Fowler, D. P. 1966. A new spruce hybrid-Picea schrenkiana x P. glauca. In Proceedings, Lake States Forest Tree Improvement Conference 7:44-47. USDA Forest Service Research Paper NC-6. North Central Forest Experiment Station, St. Paul, MN. 110 p.
- Frohlich, H. J. 1957. Die autovegetative Vermehrung durch Luftablegerverfehren. Silvae Genetica 6:143-147.
- Funsch, R. W. 1975. Spruce variation in north central Colorado. Thesis (Ph.D.), Colorado State University, Fort Collins. 79 p.
- Furniss, R. L., and V. M. Carolin. 1977. Western forest insects. U.S. Department of Agriculture, Miscellaneous Publication 1339. Washington, DC. 654 p.
- Gilgut, C. J., and 0. C. Boyd. 1933. Cytospora canker of Picea spp. Phytopathology 23(l): 11.
- Goor, A. Y., and C. W. Barney. 1976. Forest tree planting in and zones. Ronald Press, New York. 504 p.
- Griffin, G. D., and A. H. Epstein. 1964. Association of dagger nemotade, Xiphinema americanum, with stunting and winter-kill of ornamental spruce. Phytopathology 54(2):177-180.
- Hanover, James W. 1966. Inheritance of 3-carene concentration in Pinus monticola. Forest Science 12(4):447-450.
- Hanover, James W. 1974. Biochemical analysis of tree speciation. In Proceedings of the Third North American Forest Biology Workshop. [Fort Collins, CO. September 9-12, 19741 p. 106-131. C. P. P. Reid, and G. H. Fechner, eds. Colorado State University, College of Forestry and Natural Resources, Fort Collins. 388 p.
- Hanover, J. W. 1975. Genetics of blue spruce. USDA Forest Service, Research Paper WO-38. Washington, DC. 12 p.
- Hanover, J. W., and D. A. Reicosky. 1972. Accelerated growth for early testing of spruce seedlings. Forest Science 18:92-94.
- Hanover, James W., and Ronald C. Wilkinson. 1969. A new hybrid between blue spruce and white spruce. Canadian Journal of Botany 47:1693-1700.
- Hantsbarger, William, and J. W. Brewer. 1970. Insect pests of landscape plants. Cooperative Extension Service Bulletin 472A. Colorado State University, Fort Collins. 65 p.
- Hawksworth, Frank G., and John M. Staley. 1968. Rhizosphaera kalkhoffii on spruce in Arizona. Plant Disease Reporter 53(l):804-805.
- Hedlin, Alan F., Harry 0. Yates 111, David Cibrian Tovar, Bernard H. Ebel, Thomas W. Koerber, and Edward P. Merkel. 1980. Cone and seed insects of North American conifers. Canadian Forestry Service, Victoria, British Columbia. 122 p.
- Heit, C. E. 1961. Laboratory germination and recommended testing methods for 16 spruce (Picea) species. Proceedings of the Association of Official Seed Analysts 51:165-171. (Graphic Publishing, Lake Mills, IA)
- Heit, C. E. 1968. Propagation from seed: some western and exotic spruce species. American Nurseryman 127(8):12-13; 51-57;60-63.
- Hepting, George H. 1971. Diseases of forest and shade trees of the United States. U.S. Department of Agriculture, Agriculture Handbook 386. Washington, DC. 658 p.
- Hess, Karl, IV. 1981. Phyto-edaphic study of habitat types of the Arapahoe-Roosevelt National Forest, Colorado. Thesis (Ph.D.). Colorado State University, Department of Range Science, Fort Collins. 558 p.
- Jones, John R. 1973. Southwestern mixed conifers. In Silvicultural systems for the major forest types of the United States. p. 47-49. U.S. Department of Agriculture, Agriculture Handbook 445. Washington, DC.
- Jones, John R. 1974. A spot seeding trial with southwestern white pine and blue spruce. USDA Forest Service, Research Note RM-265. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 7 p.
- Jones, John R. 1974. Silviculture of the southwestern mixed conifers and aspen: the status of our knowledge. USDA Forest Service, Research Paper RM-122. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 44 p.
- Jones, John R. 1975. A southwestern mixed conifer plantation . . . case history and observations. USDA Forest Service, Research Note RM-148. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 8 p.
- Keen, F. P. 1952. (revised). Insect enemies of western forests. U.S. Department of Agriculture, Miscellaneous Publication No. 273. Washington, DC. 280 p.
- Keen, F. P. 1958. Cone and seed insects of western forest trees. U.S. Department of Agriculture, Technical Bulletin 1169. Washington, DC. 168 p.
- Kirkpatrick, Henry, Jr. 1940. Rooting evergreens with chemical. American Nurseryman 71(8):9-12.
- Kossuth, Susan V., and Gilbert H. Fechner. 1973. Incompatibility between Picea pungens Engelm. and Picea engelmannii Parry. Forest Science 19(l):50-60.
- Layser, Earle F., and Gilbert H. Schubert. 1979. Preliminary classification for the coniferous forest and woodland series of Arizona and New Mexico. USDA Forest Service, Research Paper RM-206. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 27 p.
- Leatherman, Dave. 1979. The western spruce budworm. Insects and Diseases Information Series No. 37, 2 p. Colorado State Forest Service, Fort Collins.
- Lyon, Lance. 1983. Winter grafting of cedar, spruce, and ornamental cherry. The International Plant Propagators' Society Proceedings 33:54-55.
- Mathiason, Robert L., Frank G. Hawksworth, and Carleton B. Edminster. 1986. Effects of dwarf mistletoe on spruce in the White Mountains, Arizona. Great Basin Naturalist 46(4):685-689.
- Mauk, Ronald L., and Jan A. Henderson. 1984. Forest habitat types of northern Utah. USDA Forest Service General Technical Report INT-170. Intermountain Forest and Range Experiment Station, Ogden, UT. 89 p.
- Mergen, Francois. 1958. Air-layering of Norway spruce and blue spruce. In Proceedings, Fifth Northeastern Forest Tree Improvement Conference. p. 52-54.
- Mogren, E. W. 1981. Personal communication.
- Moir, William H., and John A. Ludwig. 1979. A classification of spruce-fir mixed conifer habitat types of Arizona and New Mexico. USDA Forest Service, Research Paper RM-207. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 47 p.
- Moore, P. P., and J. W. Hanover. 1987. Variation in yield of blue spruce monoterpenes associated with crown position and frequency of resin canals. Forest Science 33(4):1081-1088.
- Nelson, D. L., and R. G. Krebill. 1970. Effect of Chrysomyxa pirolata cone rust on dispersal and viability of Picea pungens seeds. Phytopathology 600:1305.
- Nicholls, Thomas H., Allen J. Prey, and Darroll D. Skilling. 1974. Rhizosphaera kalkhoffii damages blue spruce Christmas tree plantations. Plant Disease Reporter 58(12):1094- 1096.
- Pearson, G. A. 1931. Forest types in the Southwest as determined by climate and soil. U.S. Department of Agriculture, Technical Bulletin 247. Washington, DC. 144 p.
- Peterson, R. S. 1969. Spruce broom rust. In Internationally dangerous forest tree diseases. p. 91-92. U.S. Department of Agriculture, Miscellaneous Publication 939. Washington, DC.
- Pfister, Robert Dean. 1972. Vegetation and soils in the subalpine forests of Utah. Thesis (Ph.D.). Washington State University, Pullman. 98 p.
- Phillips, Thomas W., and Gerald N. Lanier. 1983. White pine weevil, Pissodes strobi (Coleoptera: Curculionidae) attack on various conifers in New York. Canadian Entomologist 115(12):1637-1640.
- Proffer, T. J., and J. H. Hart. 1988. Vegetative compatibility groups in Leucocytospora kunzei. Phytopathology 78(3):256-260.
- Ravenstein, John. 1957. Our method of grafting blue spruce. Plant Propagation Society Proceedings 7:38-40.
- Reicosky, David A. 1974. Development and physiological effects of surface waxes on blue spruce. In Proceedings, Third North American Forest Biology Workshop. p. 374-375. C. P. P. Reid and G. H. Fechner, eds. Colorado State University, Fort Collins, CO. 388 p.
- Reicosky, David A., and James W. Hanover. 1976. Seasonal changes in leaf surface waxes of Picea pungens. American Journal of Botany 63(4):449-456.
- Safford, L. 0. 1974. Picea A. Dietr. Spruce. In Seeds of woody plants in the United States. p. 587-597. C. S. Schopmeyer, tech. coord. U.S. Department of Agriculture, Agriculture Handbook 450. Washington, DC.
- Sanderson, Peter G., and Gayle L. Worf. 1986. Phomopsis shoot blight of Colorado blue spruce. Journal of Environmental Horticulture 4(4):134-138.
- Savella, Leonard. 1965. Propagation of Picea pungens glauca cultivars. The International Plant Propagators' Society Proceedings 15:199-202.
- Schaeffer, Peter R., and James W. Hanover. 1986. Taxonomic implications of monoterpene compounds of blue and Engelmann spruces. Forest Science 32(3):725-734.
- Sherwood, Duane. 1968. Rooting of blue spruce from cuttings. The International Plant Propagators' Society Proceedings 18:187-188.
- Skilling, Darroll D. 1975. Snow blight of conifers. In Forest nursery diseases in the United States. p. 85-87. Glenn W. Peterson, and Richard S. Smith, Jr., eds. U.S. Department of Agriculture, Agriculture Handbook 470. Washington, DC. 125 p.
- Strong, W. L. 1978. Evidence of Picea pungens in north-central Montana and its significance. Canadian Journal of Botany 56(9):1118-1121.
- Sudworth, George B. 1916. The spruce and balsam fir trees of the Rocky Mountain region. U.S. Department of Agriculture, Bulletin 327. Washington, DC. 43 p.
- Tinus, R. W. 1970. Response of Pinus ponderosa Laws. and Picea pungens Engelm. seedlings to extension of photoperiod with continuous and intermittent light. Plant Physiology 46(suppl.):25.
- Tinus, Richard W. 1974. Response of several tree species to day and night temperatures. In Proceedings, Third North American Forest Biology Workshop. p. 365-388. Colorado State University, College of Forestry and Natural Resources, Fort Collins.
- Van Haverbeke, D. F. 1984. Genetic variation in blue spruce. A test of populations in Nebraska. USDA Forest Service, Research Paper RM-253. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 6 p.
- Vines, Robert A. 1960. Trees, shrubs and woody vines of the Southwest. University of Texas Press, Austin. 1104 p.
- Waterman, Alma M. 1947. Rhizosphaera kalkhoffii associated with needle cast of Picea pungens. Phytopathology 37(7):507-511.
- Watson, Gary W., and T. Davis Sydnor. 1987. The effect of root pruning on the root system of nursery trees. Journal of Arboriculture 13(5):126-130.
- Wells, James S. 1953. Propagating Koster spruce. American Nurseryman 98:13, 48-53.
- Westveld, R. H. 1939. Applied silviculture in the United States. John Wiley and Sons, Inc., New York, NY. 567 p.
- White, David A. 1975. Vegetative propagation of paired blue spruce cuttings in a liquid medium. Thesis (M.S.), Colorado State University, Fort Collins. 57 p.
- Young, Eric, and James W. Hanover. 1977. Effects of quality, intensity, and duration of light breaks during a long night dormancy in blue spruce (Picea pungens Engelm.) seedlings. Plant Physiology 60(2):271-273.

