Pinus ponderosa Dougl. ex Laws.
Ponderosa Pine
Pinaceae -- Pine family
By: William W. Oliver and Russell A. Ryker
From: Burns, Russell M., and Barbara H. Honkala, tech. coords. 1990. Silvics of North America: 1. Conifers; Agriculture Handbook 654. U.S. Department of Agriculture, Forest Service, Washington, DC. vol.2, 877 p.
Ponderosa pine (Pinus ponderosa), also called western yellow pine, is one of the most widely distributed pines in western North America. A major source of timber, ponderosa pine forests are also important as wildlife habitat, for recreational use, and for esthetic values. Within its extensive range, two varieties of the species currently are recognized: Pinus ponderosa var. ponderosa (Pacific ponderosa pine) (typical) and var. scopulorum (Rocky Mountain ponderosa pine) (10). Arizona pine (P. arizonica), sometimes classified as a variety of ponderosa pine (12,36,51), is presently recognized as a separate species (45).
Habitat
Native Range
The range of ponderosa pine extends from southern Canada into Mexico, and from the Plains States of Nebraska and Oklahoma to the Pacific Coast.
Pacific ponderosa pine (var. ponderosa) ranges from latitude 52° N. in the Fraser River drainage of southern British Columbia, south through the mountains of Washington, Oregon, and California, to latitude 33° N. near San Diego. In the northeast part of its range it extends east of the Continental Divide to longitude 110° W. in Montana, and south to the Snake River Plain, in Idaho (1,51).
Rocky Mountain ponderosa pine (var. scopulorum) extends east of the Continental Divide from latitude 48° N. in north-central Montana, southeasterly into North and South Dakota, eastern Wyoming, and as far east as north-central Nebraska. Within this area, ponderosa pine grows on the discontinuous mountains, plateaus, canyons, and breaks of the plains, with the most extensive stands found in the Black Hills of South Dakota and Wyoming (51). South of Wyoming, Rocky Mountain ponderosa pine extends south on both sides of the Continental Divide, west to Arizona, and the eastern edge of the Great Basin in Nevada, east to Texas west of the Pecos River, New Mexico, extreme northwestern Oklahoma, Colorado, and northern Mexico (36). Within this wide range, ponderosa pine is absent from a large area that includes southwestern Montana, western Wyoming, southern Idaho, and part of the Great Basin (12,61). A possible explanation for the absence is that the distribution of rainfall during the summer months prevents seedling establishment except at higher elevations, where the species has little tolerance for the shorter growing season (61).
Arizona pine (var. arizonica) is found primarily in the mountains of extreme southwestern New Mexico, southeastern Arizona, and northern Mexico (36).
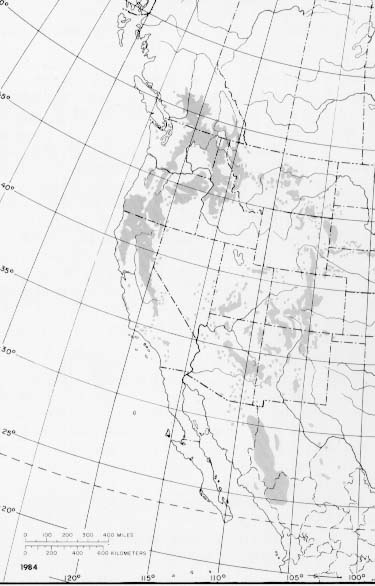
- The native range of ponderosa pine.
Climate
Throughout the range of ponderosa pine, soil moisture is the variable most often limiting growth, especially in summer when rainfall is deficient. For the east slope of the Rockies, the Black Hills, Utah, and the Southwest, however, summer rains occur, although the Southwest regularly experiences scanty May-June precipitation. In eastern Oregon and Washington, average annual precipitation ranges from 355 to 760 mm (14 to 30 in), much of it snow (30). July, August, and September are dry; average rainfall is less than 25 mm (1 in). In Montana, east of the Continental Divide, average annual precipitation in ponderosa pine forests ranges from 280 to 430 mm (11 to 17 in), with 125 to 250 mm (5 to 10 in) received during the May-to-August period (1). In the Black Hills of South Dakota, average annual precipitation is 410 to 710 mm (16 to 28 in), with up to 330 mm (13 in) received from May to August (67). In northern Arizona, 150 mm. (6 in) of the total growing season precipitation of 205 mm (8 in) occurs in July and August, after the May-June dry period. The west slope of the northern Sierra Nevada in California, where annual rainfall reaches 1750 mm (69 in), may be the wettest area supporting ponderosa pine in any quantity (13).
The extent of the seasonal rainfall deficiency is evident from the July and August precipitation, usually about 25 mm (1 in) or less; in some places, as in California, July and August precipitation is often lacking. Except on coarse-textured soils, summer showers probably provide scant moisture useful to young seedlings. Total growing season precipitation may mean little because of the distribution pattern (13).
Regardless of the location where ponderosa pine grows, average annual temperatures are between 5° and 10° C (41° and 50° F), and average July-August temperatures are between 17° and 21° C (62° and 70° F). Average frost-free seasons for ponderosa pine range from 90 to 154 days in eastern Montana and South Dakota (1,63) to more than 200 days in central California. Annual extremes are from -40° to 43° C (-40° to 110° F).
Soils and Topography
Ponderosa pine grows on soils derived from igneous, metamorphic, and sedimentary parent materials, including quartzite, argillite, schist, shale, basalt, andesite, granite, cinders, pumice, limestone, and sandstone. This results in a variety of soil orders including Entisols, Inceptisols, Mollisols, Alfisols, and Ultisols on which the species is found throughout its extensive range.
Its distribution on drier sites is related closely to supplies of available soil moisture which, in turn, are related to soil textures and depth (13,20,22). In Wyoming, for instance, at the lower limits of coniferous forest, ponderosa pine is found only on coarse-textured soils of sandstone origin where the limited moisture is more readily available than on fine-textured soils of limestone origin (27). In Oregon and Washington, higher survival and growth rates of ponderosa pine have been reported for coarse-textured sandy soils than for fine-textured clayey soils (20).
Ponderosa pine stands, 51, 75, and 78 years old, growing in coarse-, medium-, and fine-textured soils in Montana, had their greatest root development in the medium-textured soils and the least in the fine-textured soils. Root concentration was more uniform in the medium-textured soils and concentration dropped off abruptly below a soil depth of 46 cm (18 in) in the fine-textured soils (13).
Depending on the locality and the horizon of the samples, soils vary from pH 4.9 to pH 9.1. The pH in the surface horizon frequently is from 6.0 to 7.0 (13).
Foliar concentrations of nitrogen and phosphorus needed for adequate growth are low in ponderosa pine compared with the associated conifers in California-Douglas-fir (Pseudotsuga menziesii), white fir (Abies concolor), sugar pine (Pinus lambertiana), and incense-cedar (Libocedrus decurrens). Foliar concentrations of 0.9 percent for nitrogen and 0.08 percent for phosphorus mark critical boundaries between nutrient deficiency and sufficiency (42). Correcting nitrogen deficiency in California and central Oregon stands has increased volume growth 30 percent (50). Because critical levels of foliar nitrogen and phosphorus are lower in ponderosa pine, while early biomass gains generally are greater, this species is judged superior in satisfying its nutritional needs on soils that by other species' standards are infertile.
Ponderosa pine is found at elevations from sea level to 3050 m (10,000 ft). From north to south the species grows at progressively higher altitudes and within more restricted elevational limits (1, 13,20,67). In Washington, the elevations for ponderosa pine are sea level to 1220 m (4,000 ft); in the Blue Mountains of northeastern Oregon, 910 to 1520 m (3,000 to 5,000 ft); in the south-central Oregon pumice area, 1460 to 2010 m (4,800 to 6,600 ft); in the northern Rocky Mountains, from 300 to 1830 m (1,000 to 6,000 ft); in the middle Rockies up to 2590 m (8,500 ft); and in the southern Rockies, up to 3050 m (10,000 ft). In California, ponderosa pine is usually found at elevations from 150 to 1070 m (500 to 3,500 ft) in the north, and from 1610 to 2230 m (5,300 to 7,300 ft) in the south.
Associated Forest Cover
Ponderosa pine can be either a climax or a seral species (18,27,47,61). It is a climax species at the lower limits of the coniferous forests, and a seral species in higher elevation mesic forests where more competitive conifers are capable of growing. In climax forests, ponderosa pine stands often contain many small, even-aged groups rather than a true uneven-aged structure.
Fires have had a profound effect on the distribution of ponderosa pine. Although the seedlings are readily killed by fire, larger trees possess thick bark, which offers effective protection from fire damage. Competing tree species, such as grand fir (Abies grandis) and Douglas-fir, are considerably less fire tolerant, especially in the sapling and pole size classes. Ponderosa pine, therefore, was able to maintain its position as a dominant seral species on large areas of middle-elevation forests in the West. Because of successful fire control during the past 50 years, many of these stands have developed understories of Douglas-fir and true firs. Type conversion has been accelerated by harvest of the ponderosa pine, leaving residual stands composed of true fir, Douglas-fir or lodgepole pine (Pinus contorta var. latifolia) (15,20). In the Pacific Northwest, forest cover types on about 2 million ha (5 million acres) are believed to have changed in the last 25 years (3).
Ponderosa pine is an integral component of three forest cover types in the West: Interior Ponderosa Pine (Society of American Foresters Type 237), Pacific Ponderosa Pine-Douglas-Fir (Type 244), and Pacific Ponderosa Pine (Type 245) (18). Interior Ponderosa Pine is the most widespread type, covering most of the range of the species from Canada to Mexico, and from the Plains States to the Sierra Nevada, and the east side of the Cascade Mountains. Ponderosa pine is also a component of 65 percent of all western forest cover types south of the boreal forest.
Major associated tree species are as follows:
Northwest. Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca), lodgepole pine, grand fir, and western larch (Larix occidentalis).
California. California white fir (Abies concolor var. lowiana), incense-cedar, Jeffrey pine (Pinus jeffreyi), sugar pine, coast Douglas-fir (Pseudotsuga menziesii var. menziesii), California black oak (Quercus kelloggii), and western juniper (Juniperus occidentalis).
Rocky Mountains and Utah. Rocky Mountain Douglas-fir, blue spruce (Picea pungens), lodgepole pine, limber pine (Pinus flexilis), and quaking aspen (Populus tremuloides).
Black Hills. Quaking aspen, white spruce (Picea glauca), and paper birch (Betula papyrifera).
Arizona and New Mexico. White fir (Abies concolor var. concolor), Rocky Mountain Douglas-fir, blue spruce, quaking aspen, Gambel oak (Quercus gambelli), and southwestern white pine (Pinus strobiformis) at higher elevations; Rocky Mountain juniper (Juniperus scopulorum), alligator juniper (J. deppeana), and Utah juniper (J. osteosperma) at lower elevations.
Genera of understory vegetation frequently found in ponderosa pine forests are as follows:
Shrubs. Arctostaphylos, Ceanothus, Purshia, Artemisia, Quercus, Rosa, Prunus, Spiraea, Symphoricarpos, Physocarpus, and Berberis.
Grasses. Agropyron, Andropogon, Bouteloua, Festuca, Muhlenbergia, and Poa.
Community composition varies widely with geographic location, soils, elevation, aspect, and successional status. Specific information is available in descriptions of various habitat and community type classifications (1,20,23,27,35,47,61,63).
Life History
Reproduction and Early Growth
Flowering and Fruiting- Ponderosa pine is monoecious. At pollination the male strobili, borne in short, dense clusters, are 2 to 3 cm (0.8 to 1.2 in) long and female conelets are 2.5 cm (1 in) long. In western Montana, central Idaho, and eastern Oregon, at elevations from 910 to 1830 m (3,000 to 6,000 ft), flowering generally begins between May 1 and 10. Pollen is shed May 25 to June 15, cones reach a full size of 8 to 15 cm (3 to 6 in) July 20 to August 10 of the next year, seed is ripe August 20 to September 5, cones begin to open September 1 to 13, and seed is shed until November. On the east and west sides of the Sierra Nevada in California, at an elevation of 1830 m (6,000 ft), however, cones develop about 2 weeks later (13). In northern Arizona, near Flagstaff, pollen is shed between June 10 and 20 (55), but at an elevation of 910 m (3,000 ft) on the west slope of California's Sierra Nevada, pollen has been collected as early as April 15; May 11 was average for a 7-year period. Also on the west slope of the Sierra Nevada, pollen is shed an average of 8 days later for each 300 m (1,000 ft) rise in elevation (13).
In Colorado, at 2710-m (8,900-ft) elevation, during a 9-year period, female conelets emerged on or about June 18 and only about 36 percent of them survived until the beginning of the second year. Flowering is correlated closely with the passing of freezing weather (13).
Seed Production and Dissemination- No regular periodicity has been observed in the seed production of ponderosa pine over its entire range. In California, west of the Sierra Nevada, medium seed crops are borne on an average of every 2 to 3 years. The average interval between heavy cone crops is 8 years. Good cone crops are produced every 3 years in the Black Hills (7), every 3 to 4 years in the Southwest (55), and every 4 to 5 years in the Pacific Northwest (3). Observations over 23 years in Montana show ponderosa pine to be a poor seeder west, and a fair seeder east, of the Continental Divide, with only one good crop. The species bears cones as early as 7 years and continues to produce good seeds to at least 350 years. Seeds from trees aged 60 to 160, however, are more viable than those of younger or older trees. In California, trees more than 64 cm (25 in) in d.b.h. were the best producers. In central Idaho, mature and overmature trees growing at an elevation of 1680 m (5,500 ft) produced lower quality seeds than similar trees at 1220 m (4,000 ft), and open grown trees produced heavier crops of larger cones than stand grown trees (13).
In eastern Washington, Idaho, and western Montana, 16 species of insects have been identified as causing seed losses of ponderosa pine (14). They destroyed up to 95 percent of the cone crop, but most areas sampled suffered losses ranging from 30 to 60 percent. In central Arizona, abortion, ponderosa pine cone beetles (Conophthorus ponderosae), and ponderosa pine coneworms (Dioryctria sp.) were the three most important causes of cone mortality (57). Usually the proportion of seeds lost to insects is highest when crops are small.
Ponderosa pine seeds are consumed by a great many birds and small mammals such as mice, chipmunks, and tree squirrels. In years of low cone production, the potential seed crop may be severely reduced. Squirrels clip many of the cone bearing twigs, destroying flowers and conelets (13).
Specific gravity of cones containing ripe seed can be predicted. Cone collectors should consult local authorities before picking, however, because specific gravity of ripe cones varies from 0.80 in Arizona to 1.00 in the Black Hills.
The number of seeds per cone varies greatly among regions and ranges from only 31 seeds in northern Arizona (55) to 70 in central California (13). Weight of cleaned seeds varies from 15,200 to 50,700/kg (6,900 to 23,000/lb) and averages 26,500/kg (12,000/lb) (31).
Ponderosa pine seeds are not disseminated naturally over extensive distances. In central Oregon, seedfall at 37 m (120 ft) was only 22 percent of the seedfall at the west edge of a cleared area, and at 120 m (396 ft) it was only 8 percent (3). Nearly all seeds are disseminated by early November. In a good seed year as many as 852,050 seeds per hectare (345,080/acre) may reach the ground (19).
Seedling Development- Throughout ponderosa pine's range, except in the Black Hills and the west side of the Sierra Nevada, natural regeneration is sporadic. Successful natural regeneration is thought to be the result of the chance combination of a heavy seed crop and favorable weather during the next growing season. Soil texture, plant competition, and seedbed conditions are other common determinants of survival of young seedlings (13).
Germination of ponderosa pine is epigeal (31). Moisture stress reduces seed germination as well as initial seedling survival and growth. In an Arizona study, seed germination, root penetration, root dry weight, and cotyledon length decreased as the stress increased beyond 0.7 MPa (7 bars) (55). Older seedlings, however, are able to cope with limited moisture supplies by reducing transpiration and by vigorously extending their root systems. Transpiration rate declines at soil water potentials of -0.1 to -0.2 MPa (-1 to -2 bars). At -1.0 MPa (-10 bars) the transpiration rate is only 12 percent of maximum (37). Ponderosa pine has the capacity for root growth in relatively dry soil. Nursery stock lifted in January in California had appreciable root elongation even when planted in soil with a water potential of less than -0.9 MPa (-9 bars) (62) and has survived, at least for short periods, water potentials of less than -8.0 MPa (-80 bars) in the Southwest (24).
The significance of competing vegetation as a deterrent to early survival and development of young seedlings has been clearly demonstrated. In central Idaho, soil moisture remained above the wilting point at depths below 15 cm (6 in) on areas free of competing vegetation throughout the growing season but dropped to or below that critical point on most vegetated plots (13). In loamy soils in the White Mountains in Arizona, drought is normally not a major variable in seedling survival beyond age 2, except where there is grass cover (30). Shrub competition reduced the height and diameter growth of ponderosa pine planted in northern California (43); similar growth reductions have been reported for stands in Oregon (4).
Air and soil temperatures often affect growth. Seedlings grown from seed collected in Arizona, California, and South Dakota had the best root growth in 15° C (59° F) air temperature and 23° C (73° F) soil temperature. Height growth was greatest at 23° C (73° F) temperature for air and soil (33).
On the western slopes of the Sierra Nevada, height growth of ponderosa pine started significantly later with each increase of 610 m (2,000 ft) in elevation, and the length of the growing season was significantly shorter with a 910 m (3,000 ft) increase in elevation. Rates of height and radial growth did not vary with elevation during the period of growth. At an elevation of 1520 m (5,000 ft), a 6-year average showed that ponderosa pine started radial growth on March 23 and height growth on April 26. The period of radial growth lasted 177 days; that of height growth, 97 days. Ponderosa pine started height growth before sugar pine, incense-cedar, and white fir, but not before lodgepole pine (13).
Many variables cause seedling mortality. Ponderosa pine seedlings less than 36 days old were more susceptible to minimum night temperatures (lower than -5° C (23° F)) than were lodgepole pine seedlings. But by 2 months of age, differences in tolerance did not exist (8). During winters with little snow cover, 1- and 2-year-old seedlings suffered damage and killing from frost. In the Southwest, natural regeneration on fine-textured soils is almost non-existent because of frost-heaving (24). Damage is lessened by heavy cover and early summer germination of seeds, which gives a longer establishment period. Ordinarily, older seedlings are hardy in severe winter temperatures, but occasionally they suffer "winter killing" of foliage (a desiccation process) if the temperature drops suddenly when drying winds and frozen ground are present. Also, 1- to 3-month-old seedlings are killed by stem temperatures of about 54° C (130° F) and higher. Ponderosa pine is more successful in resisting high soil surface temperature with increasing age; 110-day-old seedlings can successfully withstand instantaneous temperatures of 58° to 82° C (136° to 180° F) (13). Also, it can withstand higher temperatures than its associates in the Northwest-Douglas-fir, grand fir, and Engelmann spruce (Picea engelmannii) (56).
Vegetative Reproduction- Ponderosa pine does not reproduce naturally by vegetative methods. It can be propagated by rooting and grafting, but success decreases rapidly when scions are taken from trees older than 5 years (64).
Sapling and Pole Stages to Maturity
Growth and Yield- Ponderosa pine grows to impressive size. Stems 263 cm (103.5 in) in d.b.h. and 70.7 m (232 ft) in height have been recorded (13). Diameters at breast height of 76 to 127 cm (30 to 50 in) and heights of 27.4 to 39.6 m (90 to 130 ft) are common throughout most of its range. Trees often reach ages of 300 to 600 years.
Diameter growth can be rapid and remain fairly constant for long periods provided trees are given adequate growing space. In California, on productive sites, free-growing trees can reach 66 cm (26 in) in d.b.h. in 30 years or 22 cm. (8.7 in) per decade (data on file at Pacific Southwest Forest and Range Experiment Station, Redding, CA). In central Oregon, where sites are less productive, trees 13 to 51 cm (5 to 20 in) in d.b.h. and from 19 to 36 years old can grow 12 cm (4.9 in) in d.b.h. per decade if free of intertree competition (3). Trees in a virgin stand in Arizona grew 29 mm (1.14 in) on the average during a 10-year period, but trees in a cutover stand grew 43 mm (1.68 in) (55).
Vegetative competition can restrict diameter growth markedly whether it be from neighboring trees or understory shrubs. In the central Oregon study, trees completely surrounded by understory shrubs grew only 9 cm (3.5 in) per decade. Those trees with no competitive ground cover averaged 12 cm (4.7 in) of growth per decade. In California on a droughty, skeletal soil, severe shrub competition reduced diameter growth to less than half that of competition-free trees. Insect damage, which was greater on the trees competing with shrubs, accounted for some of the growth depression (44). Stagnation in diameter, and often in height, represents a serious problem in densely stocked stands throughout the species' range, but especially on poor sites.
Height growth is most rapid in the pole and young sawtimber size classes to about 60 years. In the Pacific Northwest, dominant trees in stands of moderate density grow from 0.24 to 0.46 m (0.8 to 1.5 ft) annually between the ages of 20 to 60 years on timber-producing sites (2). Rate of growth declines gradually at older ages. Arizona trees of 160 years or older (determined at breast height) grow little in height (55). Height growth increases with site productivity and is more sensitive to stand density than was once believed.
Representative yields of ponderosa pine from a normal yield table for sites of various productivities are given in table 1 (39). For extensive natural stands, table values should be reduced by 25 percent or more because of roads, rock outcrops, steep slopes, openings, and other unproductive areas.
| Site index at base age 100 years¹ | ||||
| Age | 18 m or 60 ft | 27 m or 90 ft | 37 m or 120 ft | 46 m or 150 ft |
| yr | m³/ha | |||
| 20 | 28 | 94 | 168 | 262 |
| 40 | 122 | 238 | 396 | 588 |
| 60 | 192 | 340 | 570 | 861 |
| 80 | 238 | 413 | 696 | 1060 |
| 100 | 273 | 472 | 794 | 1204 |
| 120 | 308 | 518 | 868 | - |
| 140 | 336 | 556 | 928 | - |
| yr | ft³/acre | |||
| 20 | 400 | 1,350 | 2,400 | 3,750 |
| 40 | 1,750 | 3,400 | 5,650 | 8,400 |
| 60 | 2,750 | 4,850 | 8,150 | 12,300 |
| 80 | 3,400 | 5,900 | 9,950 | 15,150 |
| 100 | 3,900 | 6,750 | 11,350 | 17,200 |
| 120 | 4,400 | 7,400 | 12,400 | - |
| 140 | 4,800 | 7,950 | 13,250 | - |
¹Height of dominant and codominant trees of average d.b.h. |
||||
Old-growth ponderosa pine produces clear, high-grade lumber, but young trees typically are limby. Natural pruning develops slowly. An average clear length of only 3.5 m (11.5 ft) was recorded in 250-year-old stands in central Idaho (13).
Rooting Habit- The ability of ponderosa pine seedlings to grow vigorous taproots is one reason for their tenacity on severe sites where associated species often fail. Within a few months of germination, roots can penetrate to depths of 50 cm (20 in) or more in loosened and watered soil (32). This rapid root growth is essential to ponderosa pine's apparent adaptation to the climate of the Southwest. There, seeds do not germinate until the soil is continuously warm and moist. These conditions are not present until summer rains begin, usually in July. Root growth was uninhibited by grass as long as moisture was abundant (34). Taproots penetrate to about half that depth or less under average conditions in the field. Annually, for the next 2 years, lateral roots may double or triple in length.
Mature ponderosa pines put down a root to depths of more than 2 m (6 ft) in porous soils, but seldom more than 1 m (3 ft) in heavy clay soils. Exceptions occur in soils underlain by rock with deep fissures, where roots have been observed along cut banks at depths of 11 to 12 m (35 to 40 ft). In open stands, lateral roots may extend 46 m (150 ft). In dense stands, however, they are limited more to the crown width. The main mass of roots is concentrated within the top 60 cm (24 in) of the soil mantle.
Reaction to Competition- In the Sierra Nevada mixed conifer type in California, growth of advance regeneration of ponderosa pine was compared to that of associates beneath various overstory stand densities (data on file at Pacific Southwest Forest and Range Experiment Station, Redding, CA). Even beneath a light overstory stand casting 47 percent shade, ponderosa pine saplings grew only about half as rapidly as their associates (Douglas-fir, sugar pine, white fir, and incense-cedar) and about half of that expected for fully lighted pines. Relative to associates elsewhere within its range, ponderosa pine is more shade tolerant than western larch but less tolerant than grand fir and western white pine (40). Overall, it is most accurately classed as intolerant of shade.
Because of ponderosa pine's intolerance of shade, it tends to grow in even-aged stands and is usually managed by that method. Uneven-aged stands might appear common throughout the drier portion of its range but are in reality a mosaic of even-aged groups. Ponderosa pines lose vigor in dense stands. On drier sites in the Pacific Northwest, trees in pole-size stands with basal area stand densities above 34.4 m²/ha (150 ft²/acre) become subject to attack by bark beetles (54).
Ponderosa pine remains physiologically young and responds to release up to age 200 in Arizona. Elsewhere, stagnated sapling stands 70 to 100 years old usually respond to thinning and seem to grow as rapidly as unstagnated trees, when crowns grow to sufficient size to take advantage of the additional growing space (3,7).
Damaging Agents- Rabbits and hares injure or kill many seedlings, and pocket gophers are especially destructive. In areas where pocket gopher populations are high all seedlings and many saplings may be destroyed. Squirrels and porcupines attack sapling and pole-size trees and, although rarely killing them, deform the stems on which they feed. Repeated browsing by deer has stunted seedlings for 50 years or more (13,55). In the absence of regulation, sheep and cattle have damaged reproduction by trampling, bedding, and occasional browsing (13).
At least 108 species of insects attack P. ponderosa var. ponderosa, and 59 species attack P. ponderosa var. scopulorum (13). The most damaging of the tree-killing insects are several species of Dendroctonus. Trees die from the combined effects of a blue stain fungus transmitted by the beetle and extensive larval consumption of the phloem. The western pine beetle (D. brevicomis) is a common cause of mortality in overmature, decadent trees within the range of ponderosa pine from Baja California, north into Oregon, Washington, western Canada, Idaho, and western Montana. During epidemics, however, apparently healthy, vigorous trees are also killed. During the drought years of the 1930's, losses from western pine beetle in the Pacific Northwest were so heavy that many foresters feared for the pine stands' continued existence. The mountain pine beetle (D. ponderosae) is the most destructive and aggressive enemy in the central and southern Rocky Mountains. During the 1894-1908 outbreak in the Black Hills of South Dakota, this insect killed between 5.7 and 11 million m³ (1 and 2 billion/fbm) of ponderosa pine (13). Tree killing by D. ponderosae has increased with the conversion of old-growth to young-growth stands in the Pacific Northwest. High stand density is believed to reduce vigor of some of the larger trees in a stand and, therefore, is an underlying factor in the occurrence of bark beetle outbreaks. D. adjunctus, D. approximatus, and D. valens are other species of the genus that often kill ponderosa pines.
Among bark beetles, Ips species are second in destructiveness only to Dendroctonus (21). Ips are present naturally in all stands, where they usually breed in slash. In abundant slash from forestry activities, Ips can kill vigorous ponderosa pine up to 66 cm (26 in) in d.b.h. when populations reach explosive levels. Eleven species of Ips have been found attacking ponderosa pine. Of these, I. latidens, I. emarginatus, I. pini, I. lecontei, and I. paraconfusus have the most impact.
Several insects mine buds and shoots, primarily of young trees. Although seldom killed, trees are retarded in growth when infestations are severe. Pine tip moths (Rhyacionia spp.) and the gouty pitch midge (Cecidomyia piniinopis) kill the buds and shoots they mine. A more insidious pest, until recently overlooked and overrated, is the western pineshoot borer (Eucosma sonomana) (21). Larvae of this species bore within the pith of the terminal shoot, stunting but seldom killing them. Shoots that are potentially more robust are more likely to be infested than are weaker shoots. Accordingly, direct comparisons of infested vs. uninfested shoot lengths will underestimate actual growth loss. Each terminal shoot infested by a larva that developed to maturity was reduced in length that year by more than 25 percent in one study (59).
The pine reproduction weevil (Cylindrocopturus eatoni), a native of California and, presumably, Oregon, can be a threat to slow-growing plantations. Its impact has declined, however, with the improvement in planting stock and control of competing vegetation.
Defoliating insects, such as the pine butterfly (Neophasia menapia) and the pandora moth (Coloradia pandora), periodically cause damage over extensive areas. The pine needle sheathminer (Zelleria haimbachi) can be locally severe in young stands.
Dwarf mistletoe (Arceuthobium vaginatum ssp. vaginatum in the Southwest, and A. campylopodium in California and the Northwest) is ponderosa pine's most widespread disease, absent only in the Black Hills (25). It seems to be particularly devastating in the Southwest, where it infects trees on about one-third of the commercial acreage. At Fort Valley Experimental Forest in northern Arizona, dwarf mistletoe has caused up to 36 percent of the mortality (55). On trees not killed, the parasite is responsible for a significant loss in growth, primarily in height, and is reported to reduce seed viability as much as 20 percent. In the Northwest, A. campylopodium has little effect on vigorous, young trees because height growth will usually exceed its upward spread, relegating the parasite to the lower crown (5).
Several diseases attack ponderosa pine roots. Black stain root disease [Leptographium (syn. Verticicladiella) wageneri] causes a diffuse dark staining of the root wood and kills roots (6). Heterobasidion annosum causes an insidious lethal root disease that is spread by airborne spores to the surfaces of freshly-cut stumps. It and L. wageneri kill trees of all ages and usually result in group mortality that is sometimes mistaken for the work of bark beetles, which are frequently secondary invaders. Armillaria sp., previously considered weak root and butt decayers, are causing increased mortality in young plantations and thinned stands where the disease can build up in dead root systems (3). Active infection centers of L. wageneri and H. annosum spread about 1 m (3 ft) per year. The rate is usually less for Armillaria sp.
The most damaging heart rot in the southern Rocky Mountains and the Black Hills is western red rot caused by Dichomitus squalens (25). It is a major cause of loss of sound wood in commercial stands. Because ponderosa pines older than 100 years have substantially greater defect, shorter rotation ages should eliminate much of the heart rot. Phellinus pini is the major heart rot in the Pacific Coast States.
A needle cast, Elytroderma deformans, found throughout ponderosa pine's wide range, is the most serious foliage disease (6). It is unique among the needle casts in being perennial and in its capacity to infect the host twigs, which enables it to maintain its populations even under adverse environmental conditions. Although less destructive than the alarming appearance of affected trees suggests, it can slow growth and kill trees of sawtimber size. Bark beetles are prompt to attack infected trees. Severe damage from E. deformans was reported on the Ochoco National Forest in Oregon, where 555,900 m³ (98,148,000 fbm) of dying and dead trees were removed from 1946 to 1950 (13).
Several rusts of the Cronartium coleosporioides complex are damaging to ponderosa pine. Locally, especially in the Southwest, limb rust (Peridermium filamentosum) attacks middle or upper crowns of mature trees, killing branches in both directions as it spreads (46). The western gall rust (Endocronartium harknessii) attacks ponderosa pine from the Black Hills to the Pacific Northwest (25). It infects all ages, resulting in round and pear-shaped galls, distortions, and trunk lesions. Young trees may be killed. Comandra blister rust (Cronartium comandrae) is found in all states west of the Rocky Mountains but is most common in California, Idaho, Montana, Utah, and Wyoming. It causes scattered mortality in well-stocked sapling and small pole stands. In thinned stands, however, the disease may cause substantial damage (3).
Air pollution is an increasing and vexing source of foliar damage to ponderosa pine. Ozone is the major plant-damaging constituent of photochemical oxidant air pollution. Ozone becomes concentrated enough to cause damage near the border of air basins and in the predominant summer downwind direction from heavily populated areas. Because ponderosa pine, especially var. ponderosa, is susceptible, and because it grows near areas heavily polluted, ozone damage can be great. Typical injury is a chlorotic mottling accompanied by premature abscission of old needles (6). Moderately or severely injured trees are attacked more frequently by bark beetles and Heterobasidion annosum root disease (28).
Basal fire scars are common on the thick-barked stems in old-growth ponderosa pine forests. Uncontrolled fire was common before European colonization. These surface fires consumed branches, fallen trees, understory vegetation, and some living trees. The fires burned from 1 to 47 years apart, with most at 5- to 20-year intervals (3). Low-intensity fires kept many pine forests open and parklike. They also helped to maintain ponderosa pine in areas where more tolerant climax species would have attained dominance, because saplings or larger-sized ponderosa pine are more fire resistant than many of the true firs and Douglas-fir.
Survival and growth of ponderosa pine usually are affected little if 50 percent or less of the crown is scorched in a fire. Six years after a fire in Arizona, however, no poles and only 5 percent of the sawtimber-size trees were living if more than 60 percent of the crown had been destroyed (13). Low tree vigor and cambium damage increase the likelihood of mortality. Vigorous young trees have survived, on occasion, when 100 percent of their crowns were scorched. Because buds are protected by thin long scales, late season fires cause less mortality. Continued accumulation of food reserves after diameter growth ceases in late summer also increases the ability of the tree to withstand fire injury. When crowns are scorched, young, fast-growing trees on good sites have the best chance of survival and old, slow-growing trees on poor sites the poorest chance.
Snow often injures saplings and larger trees. Stem bending and breaking from unusually wet snowfalls that overload tree crowns can seriously damage dense pole-size stands (49). Stem deformation by snow pressure and movement on mountain slopes is a threat to sapling stands (38), especially where ponderosa pine is planted above its optimum elevational limit.
Special Uses
In ponderosa pine forests, timber production, livestock grazing, and recreation are the principal land uses. Ponderosa pine forests are found at low elevations offering year-round recreation, and they frequently border forest highways where esthetic values are high. They provide habitats for various wildlife species. Abert's and Kaibab squirrels usually live in the ponderosa pine forests (55). Snags in the mature pine forest provide a large number of species with nesting and roosting sites. Big game, such as deer and elk, also use the pine forests for food and shelter.
Genetics
Population Differences
Ponderosa pine shows distinct geographic variations over its widespread range. Within and between var. ponderosa and var. scopulorum, provenance studies (51,65,66) have shown genetic variation in growth, stem form, needle length, survival, initiation of leader growth, seasonal pattern of root growth potential, ability to germinate under moisture stress (41), biotic and abiotic damage (17,26,52), monoterpene production (58), nutrient status (29,68), and isozymes (10). This wealth of information on genetic diversity was summarized and interpreted recently (10). It suggests that var. ponderosa consists of three major geographic races and var. scopulorum of two major geographic races. Within var. ponderosa, the Pacific race is found in California northward from the Transverse Ranges and west of the Sierra Nevada and Cascade Range into northern Oregon. Pacific race pines have relatively large needles, cones, and seeds, and are rapid growing and least cold hardy in tests to date. The North Plateau race extends northward along the eastside of the Sierra Nevada and Cascade Range and east to the Continental Divide in Montana. Like the Pacific race, it has open, plume-like foliage, 3-needle fascicles and isozyme characteristics. But the North Plateau race has needles with thickened layers of hypoderm and sunken stomata, and is indistinguishable from the Rocky Mountain race in monoterpene characteristics. Least well understood, but distinct in monoterpene production, is the Southern California race.
Within var. scopulorum, the Rocky Mountain race comprises the northeast portion of the species' range. It is characterized by compact foliage, 2-needle fascicles, and better growth in trials east of its natural range. The Rocky Mountain race joins the Southwestern race along a broad, ill-defined front through southern Colorado, Utah, and Nevada. The Southwestern race has relatively open foliage, low proportions of 2-needle fascicles, and resins with distinctive monoterpene composition.
Results from a provenance study of 45-year-old trees in northern Idaho and a study of 30-year-old trees in Oregon and Washington (60) showed that 36 percent of the variation in the height of the trees was associated with seed source. A clinal variation was evident in the increase of height from sources in an east-to-west direction. This variation was related to September-through-June precipitation. Clinal variation in a latitudinal and altitudinal direction was related to April-May temperatures. Incidence of animal damage and of frost injury was related, also, to seed source.
Ponderosa pine varies markedly in its resistance to cold. In a test of 298 individual tree progenies planted in Michigan, all 2-year-old seedlings of California origin suffered severe injury from cold (66). Progenies from British Columbia, Washington, eastern Oregon, Arizona, and southern New Mexico suffered light damage. No damage was reported for progenies from the remainder of the species' range. Essentially the same pattern was found in the northern Idaho study in 10- to 15-year-old trees (65).
Elevational. variation has been studied intensively in central Idaho (53) and in California (9). On the west slope of the Sierra Nevada in California, seeds collected from trees growing at elevations of 40 to 2130 m (125 to 7,000 ft) were planted at altitudes of 290, 830, and 1720 m (950, 2,730, and 5,650 ft) above sea level. The general trend was that early growth was most rapid for mid-elevation sources and least rapid for high-elevation sources, regardless of the elevation of the plantation. But by 29 years, at the high-elevation plantation, sources from high elevations had overtaken sources from low elevations and had nearly caught up to sources from middle elevations. Middle and low elevation sources, especially the latter, suffered stem and leader damage from snow and wind, which significantly reduced their growth superiority. Wood specific gravity decreased with increasing elevation of parent source regardless of where the source was planted (16). No elevational effect was discerned in tracheid length, although individual differences were found. Differences were recognized, also, in total height and diameter, and in the seasonal growth pattern (42) for families within elevational zones. Genetic diversity among populations, both in California and central Idaho, was readily interpretable as adaptive variation. Results of both studies suggest that for selective breeding of a wide-ranging species with distinct elevational differentiation, such as ponderosa pine, superior progenies can be obtained from selection within the optimum elevational zone of best geographic sources. In central Idaho, the recommended elevational zone is ± 180 m (600 ft).
Hybrids
Natural crosses of ponderosa pine with Jeffrey pine have been observed in California where their ranges overlap, but they are rare. Where the two species grow in the same stand, different flowering times and other reproductive barriers restrict crossing (11). Ponderosa pine crosses with Pinus montezumae and P. arizonica, and rarely with P. engelmannii (45). Introgressive hybridization has been observed with P. washoensis.
In addition to the natural hybrids, artificial crosses have been obtained with a number of other hard pine species, including P. durangensis.
Literature Cited
- Arno, Stephen F. 1979. Forest regions of Montana. USDA Forest Service, Research Paper INT-218. Intermountain Forest and Range Experiment Station, Ogden, UT. 39 p.
- Barrett, James W. 1978. Height growth and site index curves for managed, even-aged stands of ponderosa pine in the Pacific Northwest. USDA Forest Service, Research Paper PNW-232. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 14 p.
- Barrett, James W. 1979. Silviculture of ponderosa pine in the Pacific Northwest: the state of our knowledge. USDA Forest Service, General Technical Report PNW-97. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 106 p.
- Barrett, James W. 1982. Twenty-year growth of ponderosa pine saplings thinned to five spacings in central Oregon. USDA Forest Service, Research Paper PNW-301. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 18 p.
- Barrett, James W., and Lewis F. Roth. 1985. Response of dwarf mistletoe-infested ponderosa pine to thinning: 1. Sapling growth. USDA Forest Service, Research Paper PNW-330. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 15 p.
- Bega, Robert V., tech. coord. 1978. Diseases of Pacific Coast conifers. U.S. Department of Agriculture, Agriculture Handbook 521. Washington, DC. 206 p.
- Boldt, Charles E., and James L. Van Deusen. 1974. Silviculture of ponderosa pine in the Black Hills: the status of our knowledge. USDA Forest Service, Research Paper RM-124. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 45 p.
- Cochran, P. H., and Carl M. Berntsen. 1973. Tolerance of lodgepole and ponderosa pine seedlings to low night temperatures. Forest Science 19(4):272-280.
- Conkle, M. Thompson. 1973. Growth data for 29 years from the California elevational transect study of ponderosa pine. Forest Science 19(l):31-39.
- Conkle, M. Thompson, and William B. Critchfield. 1988. Genetic variation and hybridization of ponderosa pine. In Ponderosa pine-the species and its management, symposium proceedings. p. 27-43. Washington State University, Pullman.
- Critchfield, William B. 1966. Crossability and relationships of California big-cone pines. In Proceedings, Second Genetics Workshop of Society of American Foresters and the Seventh Lake States Forest Tree Improvement Conference 1965. p. 36-44. USDA Forest Service, Research Paper NC-6. North Central Forest Experiment Station, St. Paul, MN.
- Critchfield, William B., and Elbert L. Little, Jr. 1966. Geographic distribution of the pines of the world. U.S. Department of Agriculture, Miscellaneous Publication 991. Washington, DC. 97 p.
- Curtis, James D., and Donald W. Lynch. 1965. Ponderosa pine (Pinus ponderosa Laws.). In Silvics of forest trees of the United States. p. 417-431. H. A. Fowells, comp. U.S. Department of Agriculture, Agriculture Handbook 271. Washington, DC.
- Dale, John W., and John A. Schenk. 1978. Cone production and insect-caused seed losses of ponderosa pine in Idaho and adjacent Washington and Montana. University of Idaho, Forest, Wildlife, and Range Experiment Station, Bulletin 24. Moscow. 15 p.
- Davis, Kathleen M., Bruce D. Clayton, and William C. Fisher. 1980. Fire ecology of Lolo National Forest habitat types. USDA Forest Service, General Technical Report INT-79. Intermountain Forest and Range Experiment Station, Ogden, UT. 77 p.
- Echols, R. M., and M. T. Conkle. 1971. The influence of plantation and seed-source elevation on wood specific gravity of 20-year-old ponderosa pines. Forest Science 17(3):388-394.
- Eldridge, R. H., and H. Dowden. 1980. Susceptibility of five provenances of ponderosa pine to Dothistroma needle blight. Plant Disease 64(4):400-401.
- Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Society of American Foresters, Washington, DC. 148 p.
- Foiles, Marvin W., and James D. Curtis. 1965. Natural regeneration of ponderosa pine on scarified group cuttings in central Idaho. Journal of Forestry 63(7):530-535.
- Franklin, Jerry F., and C. T. Dyrness. 1969. Vegetation of Oregon and Washington. USDA Forest Service, Research Paper PNW-80. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 216 p.
- Furniss, R. L., and V. M. Carolin. 1977. Western forest insects. U.S. Department of Agriculture, Miscellaneous Publication 1339. Washington, DC. 654 p.
- Hadley, Elmer B. 1969. Physiological ecology of Pinus ponderosa in southwestern North Dakota. American Midland Naturalist 81(2):289-314.
- Hanks, Jess P., E. Lee Fitzhugh, and Sharon R. Hanks. 1983. A habitat type classification system for ponderosa pine forests of northern Arizona. USDA Forest Service, General Technical Report RM-97. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 22 p.
- Heidmann, L. J. 1985. Ponderosa pine regeneration in the Southwest. In Proceedings of the Society of American Foresters National Convention 1985. p. 228-232. Society of American Foresters, Washington, DC.
- Hepting, George H. 1971. Diseases of forest and shade trees of the United States. U.S. Department of Agriculture, Agriculture Handbook 386. Washington, DC. 658 p.
- Hoff, R. J. 1988. Susceptibility of ponderosa pine to the needle cast fungus Lophodermium baculiferum. USDA Forest Service, Research Paper INT-386. Intermountain Research Station, Ogden, UT. 6 p.
- Hoffman, George R., and Robert R. Alexander. 1976. Forest vegetation of the Big Horn Mountains, Wyoming, a habitat type classification. USDA Forest Service, Research Paper RM-170. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 38 p.
- James, R. L., F. W. Cobb, Jr., P. R. Miller, and J. R. Parmeter, Jr. 1980. Effects of oxidant air pollution on susceptibility of pine roots to Fomes annosus. Phytopathology 70(6):560-563.
- Jenkinson, James L. 1974. Ponderosa pine progenies: differential response to ultramafic and granitic soils. USDA Forest Service, Research Paper PSW-101. Pacific Southwest Forest and Range Experiment Station, Berkeley, CA. 14 p.
- Jones, John R. 1972. Moisture stresses in Arizona mixed-conifer seedlings. USDA Forest Service, Research Paper RM-86. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 8 p.
- Krugman, Stanley L., and James L. Jenkinson. 1974. Pinus L. Pine. In Seeds of woody plants in the United States. p. 598-638. C. S. Schopmeyer, tech. coord. U.S. Department of Agriculture, Agriculture Handbook 450. Washington, DC.
- Larson, M. M. 1963. Initial root development of ponderosa pine seedlings as related to germination date and size of seed. Forest Science 9(4):456-460.
- Larson, M. M. 1967. Effect of temperature on initial development of ponderosa pine seedlings from three sources. Forest Science 13(3):286-294.
- Larson, M. M., and Gilbert H. Schubert. 1969. Root competition between ponderosa pine seedlings and grass. USDA Forest Service, Research Paper RM-54. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 12 p.
- Layser, Earle F., and Gilbert H. Schubert. 1979. Preliminary classification of the coniferous forest and woodland series of Arizona and New Mexico. USDA Forest Service, Research Paper RM-208. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 27 p.
- Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). U.S. Department of Agriculture, Agriculture Handbook 541. Washington, DC. 375 p.
- Lopushinsky, W., and G. 0. Klock. 1974. Transpiration of conifer seedlings in relation to soil water potential. Forest Science 20(2):181-186.
- Megahan, Walter F., and Robert Steele. 1987. An approach for predicting snow damage to ponderosa pine plantations. Forest Science 33(2):485-503.
- Meyer, Walter H. 1938. Yield of even-aged stands of ponderosa pine. U.S. Department of Agriculture, Technical Bulletin 630. Washington, DC. 59 p.
- Minore, Don. 1979. Comparative autecological characteristics of northwestern tree species ... a literature review. USDA Forest Service, General Technical Report PNW-87. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 72 p.
- Moore, M. B., and F. A. Kidd. 1982. Seed source variation in induced moisture stress germination of ponderosa pine. Tree Planters' Notes 33(l):12-14.
- Namkoong, G., and M. T. Conkle. 1976. Time trends in genetic control of height growth in ponderosa pine. Forest Science 22(l):2-12.
- Oliver, William W. 1979. Early response of ponderosa pine to spacing and brush: observations on a 12-year-old plantation. USDA Forest Service, Research Note PSW-341. Pacific Southwest Forest and Range Experiment Station, Berkeley, CA. 7 p.
- Oliver, William W. 1984. Brush reduces growth of thinned ponderosa pine in northern California. USDA Forest Service, Research Paper PSW-172. Pacific Southwest Forest and Range Experiment Station, Berkeley, CA. 7 p.
- Peloquin, R. L. 1984. The identification of three-species hybrids in the ponderosa pine complex. The Southwestern Naturalist 29(l):115-122.
- Peterson, Roger S. 1966. Limb rust damage to pine. USDA Forest Service, Research Paper INT-31. Intermountain Forest and Range Experiment Station, Ogden, UT. 10 p.
- Pfister, Robert D., Bernard L. Kovalchik, Stephen F. Arno, and Richard C. Presby. 1977. Forest habitat types of Montana. USDA Forest Service, General Technical Report INT-34. Intermountain Forest and Range Experiment Station, Ogden, UT. 174 p.
- Powers, Robert Field. 1981. Nutritional ecology of ponderosa pine (Pinus ponderosa Laws.) and associated species. Berkeley: The University of California. 234 p. Ph.D. dissertation.
- Powers, Robert F., and William W. Oliver. 1970. Snow breakage in a pole-sized ponderosa pine plantation ... more damage at high stand densities. USDA Forest Service, Research Note PSW-218. Pacific Southwest Forest and Range Experiment Station, Berkeley, CA. 3 p.
- Powers, Robert F., Steve R. Webster, and P. H. Cochran. 1988. Estimating the response of ponderosa pine forests to fertilization. In Proceedings-Future Forests of the Mountain West: A Stand Culture Symposium. p. 219-225. Wyman C. Schmidt, comp. USDA Forest Service, General Technical Report INT-243. Intermountain Research Station, Ogden, UT.
- Read, Ralph A. 1980. Genetic variation in seedling progeny of ponderosa pine provenances. Forest Science Monograph 23. Society of American Foresters, Washington, DC. 59 p.
- Read, Ralph A., and John A. Sprackling. 1981. Hail damage variation by seed source in a ponderosa pine plantation. USDA Forest Service, Research Note RM-410. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 6 p.
- Rehfeldt, G. E. 1986. Adaptive variation in Pinus ponderosa from Intermountain Regions. 1. Snake and Salmon River Basins. Forest Science 32(l):79-92.
- Sartwell, Charles, and Robert E. Stevens. 1975. Mountain pine beetle in ponderosa pine-prospects for silvicultural control in second-growth stands. Journal of Forestry 73(3):136-140.
- Schubert, Gilbert H. 1974. Silviculture of southwestern ponderosa pine: the status of our knowledge. USDA Forest Service, Research Paper RM-123. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 71 p.
- Seidel, K. W. 1986. Tolerance of seedlings of ponderosa pine, Douglas-fir, grand fir, and Engelmann spruce for high temperatures. Northwest Science 60(l):1-7.
- Schmid, J. M., J. C. Mitchell, and S. A. Mata. 1986. Ponderosa pine conelet and cone mortality in central Arizona. Great Basin Naturalist 46(3):445-448.
- Smith, Richard H. 1977. Monoterpines of ponderosa pine xylem resin in western United States. U.S. Department of Agriculture, Technical Bulletin 1532. Washington, DC. 48 p.
- Sower, Lonne L., and Martin D. Shorb. 1984. Effect of western pine shoot borer (Lepidoptera: Olethreutidae) on vertical growth of ponderosa pine. Journal of Economic Entomology 77(4):932-935.
- Squillace, A. E., and Roy R. Silen. 1962. Racial variation in ponderosa pine. Forest Science Monograph 2. Society of American Foresters, Washington, DC. 27 p.
- Steele, Robert, Robert D. Pfister, Russell A. Ryker, and Jay A. Kittams. 1981. Forest habitat types of central Idaho. USDA Forest Service, General Technical Report INT-114. Intermountain Forest and Range Experiment Station, Ogden, UT. 137 p.
- Stone, Edward C., and James L. Jenkinson. 1970. Influence of soil water on root growth capacity of ponderosa pine transplants. Forest Science 16(2):230-239.
- Thilenius, John F. 1971. Vascular plants of the Black Hills of South Dakota and adjacent Wyoming. USDA Forest Service, Research Paper RM-71. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO. 43 p.
- Tickner, R. L. 1969. Review of the rooting of pines. Proceedings of the International Plant Propagation Society 19:132-137.
- Wang, Chi-Wu. 1977. Genetics of ponderosa pine. USDA Forest Service, Research Paper WO-34. Washington, DC. 24 p.
- Wells, 0. 0. 1964. Geographical variation in ponderosa pine. 1. The ecotypes and their distribution. Silvae Genetics 13:89-103.
- White, E. M., J. R. Johnson, and J. T. Nichols. 1969. Prairie-forest transition soils of the South Dakota Black Hills. Proceedings Soil Science Society of America 33:932-936.
- Wright, Jonathan W., Walter A. Lemmien, and John N. Bright. 1969. Early height growth of ponderosa pine ecotypes in Michigan. Forest Science 15(2):121-129.

