Liquidambar styraciflua L.
Sweetgum
Hamamelidaceae -- Witch-hazel family
By: Paul P. Kormanik
From: Burns, Russell M., and Barbara H. Honkala, tech. coords. 1990. Silvics of North America: 2. Hardwoods; Agriculture Handbook 654. U.S. Department of Agriculture, Forest Service, Washington, DC. vol.2, 877 p.
Sweetgum (Liquidambar styraciflua), also called redgum, sapgum, starleaf-gum, or bilsted, is a common bottom-land species of the South where it grows biggest and is most abundant in the lower Mississippi Valley. This moderate to rapidly growing tree often pioneers in old fields and logged areas in the uplands and Coastal Plain and may develop in a nearly pure stand. Sweetgurn is one of the most important commercial hardwoods in the Southeast and the handsome hard wood is put to a great many uses, one of which is veneer for plywood. The small seeds are eaten by birds, squirrels, and chipmunks. It is sometimes used as a shade tree.
Habitat
Native Range
Sweetgum grows from Connecticut southward throughout the East to central Florida and eastern Texas. It is found as far west as Missouri, Arkansas, and Oklahoma and north to southern Illinois. It also grows in scattered locations in northwestern and central Mexico, Guatemala, Belize, Salvador, Honduras, and Nicaragua.
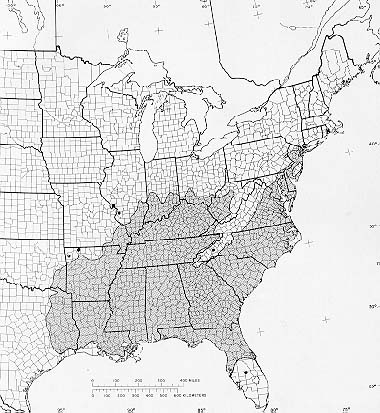
-The native range of sweetgum.
Climate
Annual rainfall varies from 1020 mm (40 in) in the North to 1520 mm (60 in) in the South; the growing season rainfall is 510 to 610 mm (20 to 24 in). There are 180 frost-free days in the northern part of its range and up to 320 in the southern part. January temperatures are less than -1° C (30° F) in the North and about 10° C (50° F) in the South; minimum temperatures during the year are -21° C (-5° F) in the North and -4° C (25° F) in the South. Maximum temperature during the year is about 38° C (100° F) for most of the range of sweetgum.
Soils and Topography
Sweetgum is perhaps one of the most adaptable hardwood species in its tolerance to different soil and site conditions. As is characteristic of most hardwood species, it grows best on the moist alluvial clay and loamy soils of river bottoms, but its growth rate is commercially acceptable on a wide range of Piedmont and Coastal Plain soils.
Throughout the Piedmont Plateau, sweetgum makes good growth on the river and stream bottoms and shows considerable potential on many upland sites. In the Carolina and Georgia Piedmont, for example, it is exceptionally competitive with other tree species on a wide range of soils with a site index for loblolly pine of 75 (at age 50) or greater.
In Maryland, sweetgum rarely makes acceptable growth on clay or gravelly clay upland soils and is rarely found on well-drained, sandy soils. Best growth rates are obtained on alluvial swamp sites and on imperfectly and poorly drained soils having a high clay content.
In the lower Mississippi Valley, site quality for sweetgum increases with the amount of exchangeable potassium in the soil and decreases as clay percentage increases. The best sites are those with medium-textured soils without a hardpan in the top 61 cm (24 in) and with moderate to good internal drainage. In the Mississippi Delta, sweetgum is most common on silty clay or silty clay loam ridges and silty clay flats in the first bottoms, which are very moist, but not too poorly drained. Along the eastern border of the Mississippi River, sweetgum is occasionally dominant on the loessial soils of the alluvial flood plain. It is characteristically dominant on the relatively impervious Alfisols of the Illinoian till plain, including the very poorly drained Avonburg, Blanchester, and Clermont silt loams (16).
Associated Forest Cover
Sweetgum is a major component of four forest cover types (6): Pin Oak-Sweetgum (Society of American Foresters Type 65), Sweetgum-Willow Oak (Type 92), Sycamore-Sweetgum-American Elm (Type 94), and Sweetgum-Yellow-Poplar (Type 87). It is a minor component of at least 20 other cover types including Chestnut Oak (Type 44), White Oak-Black Oak-Northern Red Oak (Type 52), Black Oak (Type 110), Yellow-Poplar (Type 57), River Birch-Sycamore (Type 61), Silver Maple-American Elm (Type 62), Sassafras-Persimmon (Type 64), Longleaf Pine (Type70), Longleaf Pine-Slash Pine (Type 83), Shortleaf Pine (Type 75), Virginia Pine (Type 79), Loblolly Pine (Type 81), Loblolly Pine-Shortleaf Pine (Type 80), Pond Pine (Type 98), Willow Oak-Water Oak-Diamondleaf Oak (Type 88), Sugarberry-American Elm-Green Ash (Type 93), Baldcypress Tupelo (Type 102), Water Tupelo-Swamp Tupelo (Type 103), Sweetbay-Swamp Tupelo-Redbay ('Type 104), and Cabbage Palmetto (Type 74).
Among the most common associated tree species are red maple (Acer rubrum), boxelder (A. negundo), river birch (Betula nigra), pignut, shellbark, shagbark, and mockernut hickories (Carya glabra, C. laciniosa, C. ovata, C. tomentosa), sugarberry (Celtis laevigata), shortleaf pine (Pinus echinata), and loblolly pine (P. taeda). Several species of dogwood (Cornus) and alder (Alnus), as well as eastern redbud (Cercis canadensis), commonly occur as understory species with sweetgum.
Life History
Reproduction and Early Growth
Flowering and Fruiting- Sweetgum is monoecious. The small, greenish flowers bloom from March to early May, depending on latitude and weather conditions. Both the staminate and pistillate flowers occur in heads. The staminate inflorescences are racemes; the solitary pistillate flowers are globose heads that form the multiple heads, 2.5 to 3.8 cm (1 to 1.5 in) in diameter, of small, two-celled capsules. The lustrous green color of the fruiting heads fades to yellow as maturity is reached in September to November. The beaklike capsules open at this time, and the small winged seeds, one or two per capsule, are then readily disseminated by wind. However, the seed balls can be safely collected for seed extraction several weeks before ball discoloration occurs without harming the seed. Empty fruiting heads often remain on the trees over winter. Fair seed crops occur every year and bumper crops about every 3 years. The staminate and pistillate flowers are quite sensitive to cold and are often damaged by frost (17).
Seed Production and Dissemination- Trees begin to produce seeds when 20 to 30 years old and continue production until at least 150 years of age. Seed production varies widely depending on climatic conditions during the growing season. Under optimum conditions, seed balls may average as many as 56 sound seeds per ball, or as few as 7 or 8 under less favorable conditions (16,17). Seed balls have been collected for more than 12 years at the Forestry Sciences Laboratory, Athens, GA, and scientists there expect 20 to 30 sound seeds per ball in an average year but have found as few as 5 per ball in a bad year. Low percentages of sound seed appear to be correlated with prolonged summer drought and excessive soil moisture stress during the growing season in northeast Georgia.
There are approximately 365 g (0.8 lb) of clean seeds per 35 liters (1 bushel) of balls, and the number of seeds per 454 g (1 lb) varies from 65,000 to 98,400, with an average of 82,000 (17). Seed soundness may reach 80 to 90 percent in a good seed year but may drop to 10 to 20 percent in a bad seed year. There are no data relating to the number of sound seed required for normal seed-ball development. The maximum distance of seed dispersal recorded is 183 m (600 ft), but ordinarily 96 percent of the seed falls within 61 m (200 ft) of the point of release (16).
Seedling Development- Germination is epigeal (17). Some sod covers are not a serious hindrance to seed germination but can seriously affect seedling survival during seasons of below-average rainfall. Fescue, however, has been shown to have adverse allelopathic effects on sweetgum (19). From 40 to 60 percent first-year mortality was observed on sweetgum plots overseeded with fescue in a South Carolina Piedmont site (3). The mortality at the South Carolina site was due directly to competition and was not an allelopathic response.
Vegetative Reproduction- Few data are available on the early development of natural stands of sweetgum throughout its broad range. The limited, earlier data (16) indicate that workers were not aware of the tendency of sweetgum to regenerate from root sprouts that originated from suppressed root buds (11). Stand disturbances thought to produce ideal seedbed conditions were actually optimum conditions for suppressed bud release and subsequent root sprout development. A South Carolina Coastal Plain area thought to have been successfully regenerated with sweetgum seed trees was later found to be regenerated primarily from root sprouts (4,7, 11).
The importance of root sprout formation with sweetgum regeneration is evident from observations made in natural stands of mixed pines and hardwoods in the Georgia Piedmont that have been logged for sawtimber. In most of the stands examined, advance reproduction of sweetgum was clearly evident, accounting for 10 to 60 percent of all hardwood production. The invasion of such stands by young sweetgum has usually been attributed to natural seeding, but most of the young, vigorously growing stems observed in the Georgia Piedmont were of sprout origin. It is not uncommon to find as many as 40 or more stems from seedling to sapling size on the root systems of a single parent tree. Additional work with root sprouts in the Coastal Plain of South Carolina showed that sprout height after 8 years was directly correlated with the diameter of the lateral root from which the sprout originated; the larger the root the taller the sprout.
The persistence of root sprouts was revealed when soil was removed from several 0.04-ha (0.1-acre) plots on a Georgia Piedmont bottom-land site that supported pure stands of sweetgum. Trees ranged in d.b.h. from about 25 to 41 cm (10 to 16 in) and varied from dominant to intermediate in the crown canopy. More than 70 percent of the trees were of sprout origin on most plots. Other stands that were primarily of seed origin were later found on abandoned agricultural lands. These observations indicate that a significant portion of sweetgum regeneration following logging can be expected to originate from root sprouts. The long-term development and management of these stands have yet to be clarified.
Plantation establishment of sweetgum is becoming increasingly important throughout the southern region, and it is rapidly becoming the hardwood species most commonly established. Results of early plantation establishment and development have been quite variable. This variability in growth has been attributed to seedling quality. Seedlings with a large root-collar diameter achieve the best growth, and planting seedlings with a root-collar diameter of less than 6 mm (0.25 in) is not recommended (2). In a Georgia Piedmont bottom-land site, seedlings at age 7 ranged in height from 3.8 to 6.2 in (12.4 to 20.2 ft). After 7 years on a strip mine in Indiana, sweetgum averaged 2.1 in (7 ft). On favorable sites in the lower Mississippi Valley, seedling height growth of 0.6 m/yr (2 ft/yr) has been reported. On upland sites, 5-year height growth varies considerably, from 1.1 in (3.6 ft) on an eroded field to 2.0 in (6.5 ft) on areas reverting to woody cover. It is this slow, early growth of sweetgum plantations that is of concern to silviculturists because it necessitates expensive cultivation to reduce weed competition and thereby maintain acceptable survival until height growth begins. First-order lateral root morphology of nursery-lifted sweetgum seedlings reflects their future competitiveness in the field. Early growth and survival can be acceptable, even in moderate to severe drought years, if nursery-lifted seedlings have five or more first-order lateral roots exceeding 1 mm (0.04 in) in diameter at the junction with the taproot. As many as one third of all seedlings in selected families growing in one nursery did not meet these standards making them poorly competitive in a forest environment (10).
Recent work suggests that vesicular-arbuscular mycorrhizae can significantly improve seedling quality from nurseries (9,13,14) and alter this pattern of low growth so commonly encountered during the first 3- to 5-year period following plantation establishment. On an upland Piedmont site in South Carolina, for example, total heights on sweetgum plots after three growing seasons have been observed to exceed the 2.0 in (6.5 ft) reported after five growing seasons from areas just reverting to woody cover. On a denuded borrow pit in the South Carolina Piedmont, soil amended with as little as 13 mm (0.5 in) of sewage sludge evenly distributed and disked into the soil resulted in fourth-year height of 2.8 in (9.2 ft) for sweetgum (3). The seedlings used in this experiment were heavily mycorrhizal with a vesicular-arbuscular fungus (Glomus mosseae) at outplanting.
Sapling and Pole Stages to Maturity
Growth and Yield- Young sweetgum have a strong excurrent growth habit and long, conical crowns that usually prune themselves readily under forest conditions. There is a wide range in branch angle from acute to almost 90' in young trees. Depending on site quality, and at a definite stage in development, sweetgum. becomes decurrent as the trees mature, and the crown becomes rounded and wide spreading. The tops of overmature trees are usually broken or stag headed.
The excurrent growth habit is maintained longer on the more moist, fertile bottom-land sites than on the drier, less fertile upland sites. However, on excessively dry sites the excurrent growth habit is characteristically maintained for many years and may represent a morphological growth response mediated by moisture availability.
The average 10-year diameter growth for overmature sweetgum in the southern region was reported to be 4.8 cm (1.9 in), and for immature trees of medium to high vigor, 8.9 cm (3.5 in) (16). In the Mississippi Delta, pure stands of sweetgum average 84 to 112 m³ /ha (6,000 to 8,000 fbm/acre). Very good stands have 210 to 280 m³/ha (15,000 to 20,000 fbm/acre) with up to 420 to 560 m³ /ha (30,000 to 40,000 fbm/acre) on small, selected areas. On ridges and upland sites, stands are usually less dense than on bottom-land sites.
Rooting Habit- Early root development varies with site conditions. On well-drained bottom-land sites a deep taproot with numerous well-developed laterals usually develops rapidly. On wet sites with poor drainage, however, the root system is shallow and wide spreading, with little tendency shown for taproot development. On gravelly ridges, hillsides, and upland piedmont sites, sweetgum develops a particularly strong taproot and is very resistant to wind (16).
Reaction to Competition- Sweetgum is most accurately classed as intolerant of shade. It must have adequate sunlight to reach its potential. Young sweetgum are able to endure some crowding in pure stands on bottom lands. With increasing age, however, they become less able to endure competition and may respond poorly to release because crown regeneration capacity is reduced. Sweetgum of all vigor classes tend to develop epicormic branches when stands are thinned excessively. Moderate thinnings stimulate epicormic branches, primarily on trees with light to moderate crown development (12). On upland sites in the southern and southeastern regions, sweetgum seedlings or sprouts are often present in the pine forest understory. Removal of the pine overstory usually results in rapid growth of the sweetgum. This response may be attributed to logging damage to the original understory stems, which then resprout and grow rapidly without overhead competition.
Damaging Agents- Few severe diseases are associated with sweetgum, but small mammals and grazing animals have caused isolated problems. Seedlings may be badly damaged by hogs, goats, or cattle in different areas. Rodents, particularly mice, and rabbits have caused considerable damage to young plantations in several areas (16). Beavers in the Georgia Piedmont cause impoundments and girdle healthy trees.
Fire may be one of the major agents of damage to this species. Summer fires damage young sweetgum more than winter fires. Fire scars on living trees furnish entrance points for both insects and diseases. As long as the sapwood is not killed by fire, basal wounds are often covered with a gum exudation that protects them. With repeated fires, however, a tree is apt to have some sapwood killed, and fungi and insects may become established. In the lower delta of the Mississippi River, 42 percent of the sweetgum trees burned once showed decay 8 years later; 79 percent of the trees burned repeatedly during an 8-year period showed decay (16).
The four most common decay organisms reported in the Mississippi River Delta were Fomes geotropus, Pleurotus ostreatus, Lentinus trigrinus, and Ganoderma lucidum (16).
Other diseases of sweetgum that may be important occasionally are an abiotic leader dieback or blight, twig canker, and trunk lesion caused by Botryosphaeria ribis, and bleeding necrosis, which may be a combination of sweetgum blight and B. ribis trunk lesion (8). Of these, only sweetgum blight is widely distributed and has caused heavy mortality in several States. It has received intensive study in Maryland and Mississippi. Drought appears to be the primary cause. In the lower Mississippi River flood plain, blight severity was found to be correlated with soil properties affecting moisture supply. Severity of dieback was reduced by 68 percent in 2 years by irrigating when soil moisture dropped below 40 percent of field capacity (16). There is a good possibility that sweetgum blight is most common in stands of root sprout origin. In the Georgia Piedmont and Coastal Plain of South Carolina, many groups of trees are composed of stems that are of root sprout origin and depend on a single root system complex for water uptake. During prolonged droughts such as occurred in the 1950's, this limited root system may not be adequate to satisfy the water requirements of the sprout complex, and many of the stressed trees may suffer blight.
Except for leaffeeders, insects usually attack only trees that are already damaged, decadent, or dead. These include the bark beetles (Dryocoetes betulae and Pityophthorus liquidambarus), the ambrosia beetles, which include Platypus compositus, and the darkling beetles (Strongylium spp.). The leaffeeders include the forest tent caterpillar (Malacosoma disstria) and the luna moth (Actias luna) (1). In addition, a treehopper (Strictocephala militaris) is known to spend its entire life cycle on sweetgum in northeast Georgia but is not considered to be harmful (5).
Special Uses
Sweetgum is used principally for lumber, veneer, plywood, slack cooperage, railroad ties, fuel, and pulpwood. The lumber is made into boxes and crates, furniture, radio-, television-, and phonograph cabinets, interior trim, and millwork. The veneer and plywood are used for boxes, pallets, crates, baskets, and interior woodwork (18).
Genetics
No hybrids of sweetgum are known to exist. There is considerable evidence, however, that differences between ecotypes, such as swamps and uplands, should play an important role in selection of mother trees for artificial regeneration programs (15).
Literature Cited
- Baker, Whiteford L. 1972. Eastern forest insects. U.S. Department of Agriculture, Miscellaneous Publication 1175. Washington, DC. 642 p.
- Belanger, R. P., and R. G. McAlpine. 1975. Survival and early growth of planted sweetgum related to root-collar diameter. Tree Planters'Notes 26:1, 21.
- Berry, C. R. 1981. Sewage sludge aids reclamation of disturbed forest land in the Southeast. p. 307-316. In Proceedings, Utilization of Municipal Waste Water and Sludge for Land Reclamation and Biomass Production, September 1980, Pittsburgh, PA. Environmental Protection Agency, Washington, DC.
- DeBell, D. S., 0. G. Langdon, and J. Stubbs. 1968. Reproducing mixed hardwoods by a seed-tree cutting in the Carolina Coastal Plain. Southern Lumberman 217:121-123.
- Ebel, B. H., and P. P. Kormanik. Stictocephala militaris, a membracid (Homoptera) associated with sweetgum, Liquidambar styraciflua. Annals of the Entomological Society of America 59:600-601.
- Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Society of American Foresters, Washington, DC. 148 p.
- Hook, D. D., P. P. Kormanik, and C. L. Brown. 1970. Early development of sweetgum root sprouts in Coastal South Carolina. USDA Forest Service, Research Paper SE-62. Southeastern Forest Experiment Station, Asheville, NC. 6 p.
- Hepting, George H. 1971. Diseases of forest and shade trees of the United States. U.S. Department of Agriculture, Agriculture Handbook 386. Washington, DC. 658 p.
- Kormanik, P. P. 1980. Effects of nursery practices on VA mycorrhizal development and hardwood seedling production. In Proceedings, Southern Nursery Conference, September 1980, Lake Barkley, KY. p. 63-67. Kentucky Division of Forestry and USDA Forest Service, Southeastern Area, State and Private Forestry, Atlanta, GA.
- Kormanik, P. P. 1986. Lateral root morphology as an expression of sweetgum seedling quality. Forest Science 32:595-604.
- Kormanik, P. P., and C. L. Brown. 1967. Root buds and the development of root suckers in sweetgum. Forest Science 13:338-345.
- Kormanik, P. P., and C. L. Brown. 1969. Origin and development of epicormic branches in sweetgum. USDA Forest Service, Research Paper SE-54. Southeastern Forest Experiment Station, Asheville, NC. 17 p.
- Kormanik, P. P., W. C. Bryan, and R. C. Schultz. 1977. Influence of endomycorrhizae on growth of sweetgum seedlings from eight mother trees. Forest Science 23:500-509.
- Kormanik, P. P., W. C. Bryan, and R. C. Schultz. 1977. Quality hardwood seedlings require early mycorrhizal development in nursery beds. Proceedings, Fourteenth Southeastern Forest Tree Improvement Conference. p. 289-293.
- Kormanik, P. P., W. C. Bryan, and R. C. Schultz. 1981. Effects of three vesicular-arbuscular mycorrhizal fungi on sweetgum seedlings from nine mother trees. Forest Science 27:327-335.
- U.S. Department of Agriculture, Forest Service. 1965. Silvics of forest trees of the United States. H. A. Fowells, comp. U.S. Department of Agriculture, Agriculture Handbook 271. Washington, DC. 762 p.
- U.S. Department of Agriculture, Forest Service. 1974. Seeds of woody plants in the United States. C. S. Schopmeyer, tech. coord. U.S. Department of Agriculture, Agriculture Handbook 450. Washington, DC. 883 p.
- U.S. Department of Agriculture, Forest Service. 1974. Wood handbook: wood as an engineering material. p. 1-12. U.S. Department of Agriculture, Agriculture Handbook 72, rev. Washington, DC.
- Walters, D. T., and A. R. Gilmore. 1976. Allelopathic effects of fescue on the growth of sweetgum. Journal of Chemical Ecology 2:469-479.

